ROUTINE WORK
Good larval water quality
The exact larval rearing salinity is not as critical as many early hatchery operators used to think. However, you are recommended to keep the larval cycle salinity for M. rosenbergii
BOX 8 Alternative larval stocking strategties
REARING THE LARVAE FROM STAGE I TO PL IN THE SAME TANK
If you are going to grow the larvae to metamorphosis in the same tank, stock them into the tank at 60-100 larvae/L.
TWO-STAGE REARING
If you are going to give them more space by operating a two-stage rearing method, stock them into the stage 1 tanks at about 500 larvae/L. When they reach the fifth or sixth larval development stage (see Annex 1), which takes about 10 days, reduce the density to about 50 larvae/L by transferring the larvae to other tanks.
DILUTION REARING
If you want to avoid the stress caused during the transfer of larvae to other tanks but still make the feeding more efficient and the water consumption less than in the first stocking method listed above, stock the larvae at 100 larvae/L into 35-45 cm of water at first. Then, gradually increase the water level to the normal level (70-90 cm) as the animals grow.
at 12 ppt [Note: different salinity levels apply to other Macrobrachium spp.]. Slight variations in salinity are not detrimental but you must avoid making sudden changes. These could occur, for example, if you used full strength seawater or freshwater instead of brackishwater by mistake. The simplest way to check salinity is by means of a hand-held refractometer.
The optimum temperature range for M. rosenbergii is 28-31°C. Below 24-26°C the larvae will not grow well and the time taken for them to reach metamorphosis will be longer. This affects hatchery economics enormously. Temperatures over 33°C generally cause high mortalities. Excessively high temperatures may occur when the water level is kept too low (for example, to conserve water use), especially if the tanks are outdoors and not well shaded. Gradual variation in temperature within the optimal range (such as occurs naturally between night and day or cloud and sunshine, for example) is acceptable, though it should be minimized as far as possible. Sudden changes in temperature, even as small as 1.0°C, shock the larvae and cause mortalities. It is therefore essential to have an adequate stock of prepared 12 ppt water for exchange purposes, maintained under the same environmental conditions as the larval tanks, available at all times. Do not suddenly change the larval water with water that has been in a tank standing in bright sunlight!
Dissolved oxygen levels in larval rearing water should be maintained as close as possible to saturation (Table 7). You will need to turn the aeration system off for short periods (e.g. for observation of the larvae). Double-check that you have turned the air on again immediately after any tank operation in which you have turned it off. One of the major causes of larval mortality is operator error on this point. In practice, if the procedures for water changing, tank cleaning and feeding laid down in this manual are adhered to, and there is no failure in the hatchery air distribution system, no problems should be experienced with low oxygen levels. It is not essential to measure dissolved oxygen levels in the larval rearing water, though it would be preferable to do so if a portable meter is available. This would give you a warning, before the larvae get stressed, that you need to change the water.
Keeping water quality good
The amount of organic materials, especially suspended solids, should be minimized to prevent the proliferation of heterogeneous bacteria, reduce biological oxygen demand, and prevent stress to the broodstock and larvae. Clean the tanks by siphoning excess food and waste as often as needed. Many invisible changes in the chemical water quality of larvalrearing water occur. These are due mainly to the metabolic wastes produced by the larvae themselves (and by live feeds) and by the degradation of excess food. Some of these changes can be extremely harmful to larvae. The most serious are increases in the non-ionized8 form of ammonia (NH3), which is especially evident at high pH and temperature, and in nitrite. It is beyond the scope of this manual to deal with water chemistry but those who wish to study this matter should consult the review by Valenti and Daniels (2000), which also contains references to other publications on this topic.
If you are running a hatchery based on the flow-through ‘clearwater’ system, there is no substitute for frequent water exchange. Recommended procedures to maintain good larval water quality in flow-through systems are given in Box 9. Further recommendations on system hygiene are made later in this manual.
Special considerations for recirculation systems
Routine care is even more essential in recirculation than in flow-through hatcheries, esp
BOX 9
Recommendations for good larval water quality
DO NOT OVERFEED. Maintain good hygiene and clean the inner surfaces of the tanks every two days by means of a ‘squeegee’ or scraper.
Turn off the air supply to allow solid particles to settle, and siphon off (Figures 34a and 34b) surplus food particles and metabolic wastes from the bottom of the tank. Do this daily, immediately before one of the feeding operations. Keep the time taken to complete this task to a minimum and turn the air on again as soon as possible.
Make this part of the daily water exchange procedure. Siphoning will also remove any mortalities which have occurred. This provides you with a good opportunity to observe the condition of your larvae.
There is no great danger of losing healthy animals while siphoning because the larvae swim in the body of the water and do not crawl. There may be some live larvae on the bottom of the tank and these may pass through the siphon tube. Some hatchery operators collect these (Figure 35) and return them to the larval tank. You are not recommended to do this. Discard these larvae because they are probably too weak to evade the oncoming siphon tube and are therefore of poor quality.
Never hesitate to exchange the larval water (in addition to routine water exchange, see below) at any time you suspect that the water is poor. Poor water quality (due, for example, to excessive overfeeding) can be detected if the dissolved oxygen level is low, if the water appears turbid and/or smells foul, or the animals appear in ‘poor condition’.
Larvae in ‘poor condition’ are sluggish, not active. They do not appear to be strong enough to swim against the air bubbles, are found only at the edges of the tank, and sometimes jump out of the tank. Non-feeding larvae can be detected by their colour. Normally they should be brownish, due to the consumption of brine shrimp nauplii. If you are worried about poor water quality, immediately change most of the water, taking care to replace it with water of the correct salinity and temperature.
Operate the turn-down drain until the water depth is only about 10 cm, flush the tank with ‘new’ water for 10-15 minutes and then fill up to 70 cm again. The ‘new’ water used for flushing and replacement must be pre-aerated, 12 ppt salinity, and the same temperature as the ‘old’ water, and the tank aeration system must be kept on throughout the operation.
Regularly exchange some of the water in the tank, according to its quality (see above). You should not have to change it during the first three or four days of the larval rearing cycle. Then, as you begin introducing inert food, exchange 50% of the water every day, or every second day, according to its quality. You may find it necessary to increase the exchange rate to over 50% per day toward the end of the rearing cycle, when biomass and feeding levels are at their greatest. Decrease the water level from 70 cm to about 35 cm, partly through the siphoning operation described above, and partly by use of the turn-down drain. Replace the water removed with readymixed, aerated, 12 ppt water at the same temperature as that already in the larval tank. Do this operation before feeding, so that food will not be wasted.
You may find it useful to follow the practice of some freshwater prawn and marine shrimp hatcheries that maintain 10 ppm of the sodium salt of ethylene diaminetetraacetic acid (EDTA) in the larval rearing water, believing it to improve productivity because of its chelating ability (see glossary – Annex 11).
cially when the filtration system is new. Despite this, those familiar with recirculation systems claim that the amount of labour required is not greater than in flow-through systems.
Following the suggestions of Valenti and Daniels (2000), a routine maintenance schedule for recirculation systems is suggested in Box 10. Further recommendations on system hygiene are made later in this manual.
FIGURE34a
Tanks need regular siphoning to remove faeces, the larval exoskeletons that are cast off during moulting, and waste food
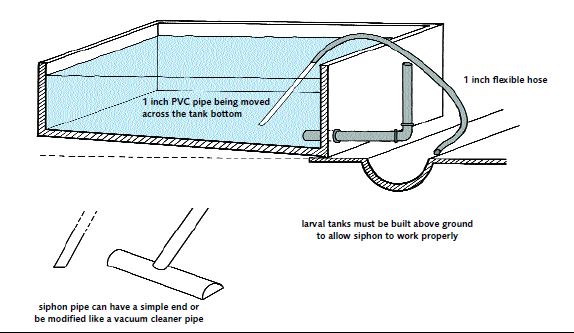
Figure 34b
Good tank hygiene is essential for hatchery success (Hawaii)

A detailed description of a recirculation freshwater prawn hatchery is provided in Daniels, D’Abramo and Parseval (1992) and Fuller, Kelly and Smith (1992). Further reading is provided in Valenti and Daniels (2000). A simple recirculation system is described in Chowdhury, Bhattacharjee and Angell (1993).