3.2 THE FIVE MOST IMPORTANT WATER QUALITY PARAMETERS
3.2.1 Oxygen
Oxygen is essential for all three organisms involved in aquaponics; plants, fish and nitrifying bacteria all need oxygen to live. The DO level describes the amount of molecular oxygen within the water, and it is measured in milligrams per litre. It is the water quality parameter that has the most immediate and drastic effect on aquaponics.
Indeed, fish may die within hours when exposed to low DO within the fish tanks. Thus, ensuring adequate DO levels is crucial to aquaponics. Although monitoring DO levels is very important, it can be challenging because accurate DO measuring devices can be very expensive or difficult to find. It is often sufficient for small-scale units to instead rely on frequent monitoring of fish behaviour and plant growth, and ensuring water and air pumps are constantly circulating and aerating the water.
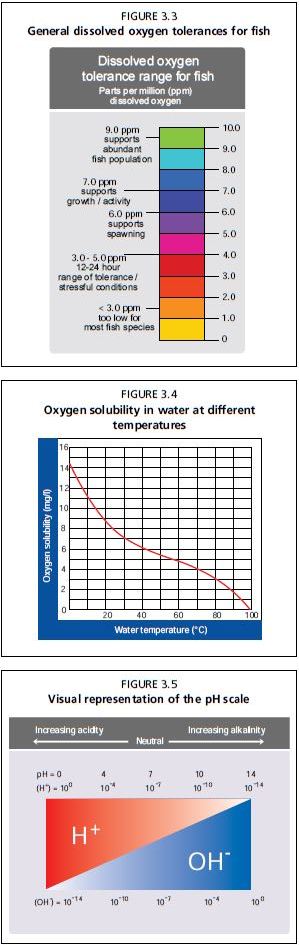
Oxygen dissolves directly into the water surface from the atmosphere. In natural conditions, fish can survive in such water, but in intensive production systems with higher fish densities, this amount of DO diffusion is insufficient to meet the demands of fish, plants and bacteria. Thus, the DO needs to be supplemented through management strategies. The two strategies for small-scale aquaponics are to use water pumps to create dynamic water flow, and to use aerators that produce air bubbles in the water. Water movement and aeration are critical aspects of every aquaponic unit, and their importance cannot be overstressed. These topics, including methods of design and redundancy, are discussed further in Chapter 4. The optimum DO levels for each organism to thrive are 5-8 mg/litre (Figure 3.3). Some species of fish, including carp and tilapia, can tolerate DO levels as low as 2-3 mg/litre, but it is much safer to have the levels higher for aquaponics, as all three organisms demand the use of the DO in the water.
Water temperature and DO have a unique relationship that can affect aquaponic food production. As water temperature rises, the solubility of oxygen decreases. Put another way, the capacity of water to hold DO decreases as temperature increases; warm water holds. less oxygen than does cold water (Figure 3.4). As such, it is recommended that aeration be increased using air pumps in warm locations or during the hottest times of the year, especially if raising delicate fish.
3.2.2 PH
A general knowledge of pH is useful for managing aquaponic systems. The pH of a solution is a measure of how acidic or basic the solution is on a scale ranging from 1 to 14. A pH of 7 is neutral; anything below 7 is acidic, while anything above 7 is basic. The term pH is defined as the amount of hydrogen ions (H) in a solution; the more hydrogen ions, the more acidic.
Two important aspects of the pH scale are illustrated in Figure 3.5.
The pH scale is negative; a pH of 7 has fewer hydrogen ions than a pH of 6.
The pH scale is logarithmic; a pH of 7 has 10 times fewer hydrogen ions than a pH of 6, 100 times fewer than a pH of 5, and 1000 times fewer than a pH of 4. For example, if the pH of an aquaponic unit is recorded as 7, and later the value is recorded as 8, the water now has ten times fewer freely associated H+ ions because the scale is negative and logarithmic. It is important to be aware of the logarithmic nature of the pH scale because it is not necessarily intuitive. For the previous example, if a later reading showed the pH to be 9, the problem would be 100 times worse, and therefore hypercritical, instead of just being two times worse.
Importance of pH
The pH of the water has a major impact on all aspects of aquaponics, especially the plants and bacteria. For plants, the pH controls the plants' access to micro- and macronutrients. At a pH of 6.0-6.5, all of the nutrients are readily available,
but outside of this range the nutrients become difficult for plants to access. In fact, a pH of 7.5 can lead to nutrient deficiencies of iron, phosphorus and manganese. This phenomenon is known as nutrient lock-out and is discussed in Chapter 6.
Nitrifying bacteria experience difficulty below a pH of 6, and the bacteria's capacity to convert ammonia into nitrate reduces in acidic, low pH conditions. This can lead to reduced biofiltration, and as a result the bacteria decrease the conversion of ammonia to nitrate, and ammonia levels can begin to increase, leading to an unbalanced system stressful to the other organisms.
Fish have specific tolerance ranges for pH as well, but most fish used in aquaponics have a pH tolerance range of 6.0-8.5. However, the pH affects the toxicity of ammonia to fish, with higher pH leading to higher toxicity. This concept is more fully discussed in the Section 3.4. In conclusion, the ideal aquaponic water is slightly acidic, with an optimum pH range of 6-7. This range will keep the bacteria functioning at a high capacity, while allowing the plants full access to all the essential micro- and macronutrients. pH values between 5.5 and 7.5 require management attention and manipulation through slow and measured means, discussed in Section 3.5 and in Chapter 6. However, a pH lower than 5 or above 8 can quickly become a critical problem for the entire ecosystem and thus immediate attention is required.
There are many biological and chemical processes that take place in an aquaponics system that affect the pH of the water, some more significantly than others, including: the nitrification process; fish stocking density; and phytoplankton.
The nitrification process
The nitrification process of bacteria naturally lowers the pH of an aquaponic system. Weak concentrations of nitric acid are produced from the nitrification process as the bacteria liberate hydrogen ions during the conversion of ammonia to nitrate. Over time, the aquaponic system will gradually become more acidic primarily as a result of this bacterial activity.
Fish stocking density
The respiration, or breathing, of the fish releases carbon dioxide (CO2) into the water. This carbon dioxide lowers pH because carbon dioxide converts naturally into carbonic acid (H2CO3) upon contact with water. The higher the fish stocking density of the unit, the more carbon dioxide will be released, hence lowering the overall pH level. This effect is increased when the fish are more active, such as at warmer temperatures.
Phytoplankton
Respiration by fish lowers the pH by releasing carbon dioxide into the water, conversely, the photosynthesis of plankton, algae and aquatic plants remove carbon dioxide from the water and raises the pH. The effect of algae on pH follows a daily pattern, where the pH rises during the day as the aquatic plants photosynthesize and remove carbonic acid, and then falls overnight as the plants respire and release carbonic acid. Therefore, the pH is at a minimum at sunrise and a maximum at sunset. In standard RAS or aquaponic systems, phytoplankton levels are usually low and, therefore, the daily pH cycle is not affected. However, some aquaculture techniques, such as pond aquaculture and some fish breeding techniques, deliberately use phytoplankton, so the time of monitoring should be chosen wisely.
3.2.3 Temperature
Water temperature affects all aspects of aquaponic systems. Overall, a general compromise range is 18-30 °C. Temperature has an effect on DO as well as on the toxicity (ionization) of ammonia; high temperatures have less DO and more unionized (toxic) ammonia. Also, high temperatures can restrict the absorption of calcium in plants. The combination of fish and plants should be chosen to match the ambient temperature for the systems' location, and changing the temperature of the water can be very energy-intensive and expensive. Warm-water fish (e.g. tilapia, common carp, catfish) and nitrifying bacteria thrive in higher water temperatures of 22-29 °C, as do some popular vegetables such as okra, Asian cabbages, and basil. Contrarily, some common vegetables such as lettuce, Swiss chard and cucumbers grow better in cooler temperatures of 18-26 °C, and cold-water fish such as trout will not tolerate temperatures higher than 18 °C. For more information on optimal temperature ranges for individual plants and fish, see Chapters 6 and 7 on plant and fish production, respectively, and Appendix 1 for key growing information on 12 popular vegetables.
Although it is best to choose plants and fish already adapted to the local climate, there are management techniques that can minimize temperature fluctuations and extend the growing season. Systems are also more productive if the daily, day to night, temperature fluctuations are minimal. Therefore, the water surface itself, in all of the fish tanks, hydroponic units and biofilters, should be shielded from the sun using shade structures. Similarly, the unit can be thermally protected using insulation against cool night temperatures wherever these occur. Alternatively, there are methods to passively heat aquaponic units using greenhouses or solar energy with coiled agricultural pipes, which are most useful when temperatures are lower than 15 °C; these methods are described in more detail in Chapters 4 and 9.
It is also possible to adopt a fish production strategy to cater for temperature differences between winter and summer, particularly if the winter season has average temperatures of less than 15 °C for more than three months. Generally, this means cold-adapted fish and plants are grown during winter, and the system is changed over to warm-water fish and plants as the temperatures climb again in spring. If these methods are not feasible during cold winter seasons, it is also possible to simply harvest the fish and plants at the start of winter and shut down the systems until spring. During summer seasons with extremely warm temperatures (more than 35 °C), it is essential to select the appropriate fish and plants to grow (see Chapters 6 and 7) and shade all containers and the plant growing space.
3.2.4 Total nitrogen: ammonia, nitrite, nitrate
Nitrogen is the fourth crucial water quality parameter. It is required by all life, and part of all proteins. Nitrogen originally enters an aquaponic system from the fish feed, usually labelled as crude protein and measured as a percentage. Some of this protein is used by the fish for growth, and the remainder is released by the fish as waste. This waste is mostly in the form of ammonia (NH3) and is released through the gills and as urine. Solid waste is also released, some of which is converted into ammonia by microbial activity. This ammonia is then nitrified by bacteria, discussed in Section 2.1, and converted into nitrite (NO2) and nitrate (NO3). Nitrogenous wastes are poisonous to fish at certain concentrations, although ammonia and nitrite are approximately 100 times more poisonous than nitrate. Although toxic to fish, nitrogen compounds are nutritious for plants, and indeed are the basic component of plant fertilizers. All three forms of nitrogen (NH3, NO, and NO3) can be used by plants, but nitrate is by far the most accessible. In a fully functioning aquaponic unit with adequate biofiltration, ammonia and nitrite levels should be close to zero, or at most 0.25-1.0 mg/litre. The bacteria present in the biofilter should be converting almost all the ammonia and nitrite into nitrate before any accumulation can occur.
Impacts of high ammonia
Ammonia is toxic to fish. Tilapia and carp can show symptoms of ammonia poisoning at levels as low as 1.0 mg/litre. Prolonged exposure at or above this level will cause damage to the fishes' central nervous system and gills, resulting in loss of equilibrium, impaired respiration and convulsions. The damage to the gills, often evidenced by red colouration and inflammation on the gills, will restrict the correct functioning of other physiological processes, leading to a suppressed immune system and eventual death. Other symptoms include red streaks on the body, lethargy and gasping at the surface for air. At higher levels of ammonia, effects are immediate and numerous deaths can occur rapidly. However, lower levels over a long period can still result in fish stress, increased incidence of disease and more fish loss.
As discussed above, ammonia toxicity is actually dependent on both pH and temperature, where higher pH and water temperature make ammonia more toxic. Chemically, ammonia can exist in two forms in water, ionized and unionized. Together, these two forms together are called total ammonia nitrogen (TAN), and water testing kits are unable to distinguish between the two. In acidic conditions, the ammonia binds with the excess hydrogen ions (low pH means a high concentration of H) and becomes less toxic. This ionized form is called ammonium. However, in basic conditions (high) pH, above 7), there are not enough hydrogen ions and the ammonia remains in its more toxic state, and even low levels of ammonia can be highly stressful for the fish. This problem is exacerbated in warm water conditions.
Activity of nitrifying bacteria declines dramatically at high levels of ammonia. Ammonia can be used as an antibacterial agent, and at levels higher than 4 mg/litre it will inhibit and drastically reduce the effectiveness of the nitrifying bacteria. This can result in an exponentially deteriorating situation when an undersized biofilter is overwhelmed by ammonia, the bacteria die and the ammonia increases even more.
Impacts of high nitrite
Nitrite is toxic to fish. Similar to ammonia, problems with fish health can arise with concentrations as low as 0.25 mg/litre. High levels of NO2 can immediately lead to rapid fish deaths. Again, even low levels over an extended period can result in increased fish stress, disease and death.
Toxic levels of NO2 prevent the transport of oxygen within the bloodstream of fish, which causes the blood to turn a chocolate-brown colour and is sometimes known as "brown blood disease". This effect can be seen in fish gills as well. Affected fish exhibit similar symptoms to ammonia poisoning, particularly where fish appear to be oxygen- deprived, seen gasping at the surface even in water with a high concentration of DO. Fish health is covered in more detail in Chapter 7.
Impacts of high nitrate
Nitrate is a far less toxic than the other forms of nitrogen. It is the most accessible form of nitrogen for plants, and the production of nitrate is the goal of the biofilter. Fish can tolerate levels of up to 300 mg/litre, with some fish tolerating levels as high as 400 mg/litre. High levels (> 250 mg/litre) will have a negative impact on plants, leading to excessive vegetative growth and hazardous accumulation of nitrates in leaves, which is dangerous for human health. It is recommended to keep the nitrate levels at 5-150 mg/litre and to exchange water when levels become higher.
3.2.5 Water hardness
The final water quality parameter is water hardness. There are two major types of hardness: general hardness (GH), and carbonate hardness (KH). General hardness is a measure of positive ions in the water. Carbonate hardness, also known as alkalinity, is a measure of the buffering capacity of water. The first type of hardness does not have a major impact on the aquaponic process, but KH has a unique relationship with pH that deserves further explanation.
General hardness
General hardness is essentially the amount of calcium (Ca2+), magnesium (Mg) and, to a lesser extent, iron (Fe) ions present in water. It is measured in parts per million (equivalent to milligrams per litre). High GH concentrations are found in water. sources such as limestone-based aquifers and/or river beds, as limestone is essentially composed of calcium carbonate (CaCO3). Both Ca2+ and Mg2+ ions are essential plant nutrients, and they are taken up by plants as the water flows through the hydroponic components. Rainwater has low water hardness because these ions are not found in the atmosphere. Hard water can be a useful source of micronutrients for aquaponics, and has no health effects on the organisms. In fact, the presence of calcium in the water can prevent fish from losing other salts and lead to a healthier stock.
Carbonate hardness or alkalinity
Carbonate hardness is the total amount of carbonates (CO,2) and bicarbonates (HCO3) dissolved in water. It is also measured in milligrams of CaCO, per litre. In general, water is considered to have high KH at levels of 121-180 mg/litre. Water sourced from limestone bedrock wells/aquifers will normally have a high carbonate hardness of about 150-180 mg/litre.
Carbonate hardness in water has an impact on the pH level. Simply put, KH acts as a buffer (or a resistance) to the lowering of pH. Carbonate and bicarbonate present in the water will bind to the H+ ions released by any acid, thus removing these free H+ ions from the water. Therefore, the pH will stay constant even as new H+ ions from the acid are added to the water. This KH buffering is important, because rapid changes in pH are stressful to the entire aquaponic ecosystem. The nitrification process generates nitric acid (HNO3), as discussed in Section 3.2.2, which is dissociated in water in its two components, hydrogen ions (H) and nitrate (NO3), with the latter used as source of nutrients for plants. However, with adequate KH the water does not actually become more acidic. If no carbonates and bicarbonates were present in the water, the pH would quickly drop in the aquaponic unit. The higher the concentration of KH in the water, the longer it will act as a buffer for pH to keep the system stable against the acidification caused by the nitrification process.
The next section describes this process in more detail. It is a rather complicated process but it is important to understand for aquaponics (or other soil-less culture) practitioners where the available water is naturally very hard (which is normally the case in regions with limestone or chalk bedrock), as pH manipulation will become a vital part of unit management. Section 3.5 contains specific techniques of pH manipulation. The summary following the extended description will list what is essential for all practitioners to know regarding hardness.
As mentioned above, the constant nitrification in an aquaponic unit produces nitric acid and increases the number of H+ ions, which would lower the pH in the water. If no carbonates or bicarbonates are present to buffer the H+ ions in the water, the pH will quickly drop as more H+ ions are added into the water. Carbonates and bicarbonates, as shown in Figure 3.6, bind the hydrogen ions (H+) released from the nitric acid and maintain a constant pH by balancing the surplus of H+ with the production of carbonic acid, which is a very weak acid. The H+ ions remain bound to the compound and are
FIGURE 3.6
Hydrogen and carbonate ions bonding
H+ CO32=> HCO3 & H++ HCO3 => H2CO3
(Free hydrogen) (Carbonate) (Bicarbonate) (Free hydrogen) (Bicarbonate) (Carbonic acid)
FIGURE 3.7 Bicarbonate and nitric acid bonding in aquaponics
HNO3 + HCO3 => H2CO3 + NO3 (Carbonic acid) (Nitrate)
(Nitric acid) (Bicarbonate)
not free in the water. Figure 3.7 shows in more detail the bonding process occurring with nitric acid.
It is essential for aquaponics that a certain concentration of KH is present at all times in the water, as it can neutralize the acids created naturally and keep the pH constant. Without adequate KH, the unit could be subjected to rapid pH changes that would have negative impacts on the whole system, especially the fish. However, KH is present in many water sources. Replenishing the unit with water from these sources will also replenish the levels of KH. However, rainwater is low in KH, and in rainfed systems it is helpful to add external sources of carbonate, as explained below.
Summary of essential points on hardness
General Hardness (GH) is the measurement of positive ions, especially calcium and magnesium.
Carbonate Hardness (KH) measures the concentration of carbonates and bicarbonates that buffer the pH (create resistance to pH change). Hardness can be classified along the water hardness scale as shown below:
The optimum level of both hardness types for aquaponics is about 60-140 mg/litre. It is not vital to check the levels in the unit, but it is important that the water being used
Water hardness classification. mg/litre
soft 0-60 mg/litre
moderately hard 60-120 mg/litre
hard 120-180 mg/litre
very hard > 180 mg/litre
to replenish the unit has adequate concentrations of KH to continue neutralizing the nitric acid produced during the nitrification process and to buffer the pH at its optimum level (6-7).