5.1.3 Methods for rearing larvae
D-larvae recovered are counted as described above. They are now at the stage where they need feeding with unicellular, cultured algae. Species of good nutritional value include the diatoms,
Chaetoceros calcitrans,
Chaetoceros muelleri,
Thalassiosira pseudonana (3h)
and the flagellates,
Isochrysis galbana (or the ‘T-Iso’ clone),
Part 5 - Hatchery operation: culture of larvae 93
Pavlova lutherii, and one of the
Tetraselmis species (but only for larvae >120 ?m length).
Details of diets, rations and how to calculate them are described in part 5.2
Larvae can be grown in the same flat-bottomed tanks used for embryo development or in conical-based fibreglass tanks fitted with bottom drains (see Figure 52). Tanks may be of relatively small volume (200 to 1 000 l) for experimental purposes and small volume production or much greater in both size and volume in high volume output commercial hatcheries. They may be operated as static systems or on flow-through. Water is changed in static systems on a periodic basis, whereas in flow-through culture, it is continuously introduced, a fixed volume being exchanged and replaced daily. This topic is discussed in depth in section 5.1.4.2.
D-larvae of hardier species (including Crassostrea and Tapes) can be grown at densities of 15 000 to 20 000 per l, but growth and survival are generally improved at lower densities (Table 10). Reduced densities are recommended for scallop species of the genera Pecten, Patinopecten, Placopecten and species of Chlamys and Argopecten, where between 5 000 and 10 000 early-stage larvae per l is appropriate. The larviparous flat oyster, Ostrea edulis is generally grown at 2 000 to 5 000 larvae per l because of the large size of the intial D-larvae. Some species can be successfully grown more intensively than above using high-density culture techniques (see section 5.1.4.1).
Rearing tanks are aerated – most commonly by a single, central air outlet located just off the tank bottom – at flow rates ranging from a slow bubble rate for D-larvae increasing to 200 l per hour for later stage larvae. The source of pressurized air needs to be free of carbon and oil. Low pressure, high volume, regenerative air blowers are ideal for the purpose. The air is filtered at source to 0.22 or 0.45 ?m particle size by a series of cartridge filters of decreasing porosity. This is to reduce air-borne contaminants which may include harmful micro-organisms. It is also advisable in humid conditions to dry the air before it enters the tanks by passing it through a sealed unit, such as filter
cartridge housings, containing either anhydrous calcium chloride or silica gel. These drying agents need to be replaced as they become saturated to be effective.
5.1.3.1 Starting a new culture
Larval culture tanks and all equipment to be used must be thoroughly cleaned and then rinsed with either freshwater or filtered seawater. Mild liquid detergents added to hot water, or suitably diluted sterilizing/disinfecting agents such as bleach (sodium hypochlorite solution) at 20 mg per l free-chlorine can be used for cleaning purposes. The process of starting a new culture is as follows:
a) Fill the required number of clean larval rearing tanks with seawater filtered to 1 or 2 ?m at the required temperature and salinity.
Note: It may be beneficial to reduce near oceanic salinities when rearing euryhaline species such as the American eastern oyster, C. virginica, by adding finely filtered freshwater from a clean, unpolluted source. A salinity of between 20 and 25 psu is recommended for this and other Crassostrea species).
b) If problems have been experienced with abnormal mortality of larvae in the recent past, bacteria may have been responsible. In this situation UV-light treatment of the water before filling the tanks is used by some hatcheries and may help. As a last resort if mortalities persist, a broad-spectrum antibiotic such as chloramphenicol at 2-5 mg per l of seawater may be used experimentally under veterinary prescription.
c) Add D-larvae to the vessel at the appropriate density.
d) Calculate, following the procedure given in part 5.2.3.2, The volumes of harvested algae to add to the vessels to provide the required food ration.
e) Turn on the flow of air so that there is a good turnover of water to suspend and evenly mix both the larvae and food.
f) The culture is then left for 24 hours before further husbandry is necessary.
5.1.3.2 Husbandry of larval cultures
Cultures of larvae require daily maintenance. They are operated most commonly as static water systems, i.e. without a continuous exchange of water, although some hatcheries operate flow-through culture systems (see section 5.1.4.2). Food cell concentrations need to be maintained at levels conducive to efficient feeding activity.
To prevent the accumulation of potentially harmful, metabolites, tanks require complete water changes at regular intervals throughout larval development from the D-stage to the onset of metamorphosis. The frequency with which this is done depends on the number and mean size of larvae being cultured. Water is changed either at 48-hour intervals or 3 times each week:
- at higher densities of early-stage larvae (15 000 to 20 000 per l at <120 ?m), - at low densities of late-stage larvae (<5 000 per litre at 150 to 200 ?m) or , - at about 2 000 per litre at 250 to 300 ?m).
Note: The values given are a rough guide to applicable densities related to mean shell length. More accurately, if a culture requires feeding less than 200 cells per ?l Isochrysis equivalents per day then it is regarded as low density – see part 5.2.3.1). Water changes need to be daily at higher daily additions of food or alternatively, the tanks operated on the flow-through principle.
(i) Husbandry on non water change day
Husbandry on days between water changes consists of restoring the food cell concentration to compensate for cells eaten in the previous 24-h period by adding sufficient freshly harvested algae. A sample of water is taken from each vessel and the remaining algae cells (residual algae) per unit volume are counted, either by using a microscope with a haemocytometer slide at x100 magnification, or, more easily, by a coulter counter or similar particle counter. Where it is impractical to determine residual algae, either a full or partial ration of food can be added on intermediate days between water changes based on the previous day’s ration fed.
Daily records should be kept of culture temperatures, residual algae and any additions of food to restore optimum food cell concentrations. An example of such a record form is shown in Figure 60. Volumes of additional algae are calculated as described in part 5.2.3.2.
(ii) Husbandry on water change days
The procedure is similar to that described and illustrated in the previous section on embryo development (Figure 56). The tank is emptied by means of a siphon or from a bottom drain, delivering the discharge flow into a sieve which is of sufficient aperture to retain large debris but not the larvae – a 250 ?m sieve is ideal (Figure 61). The larvae are retained on the mesh of a lower sieve of suitable mesh aperture.
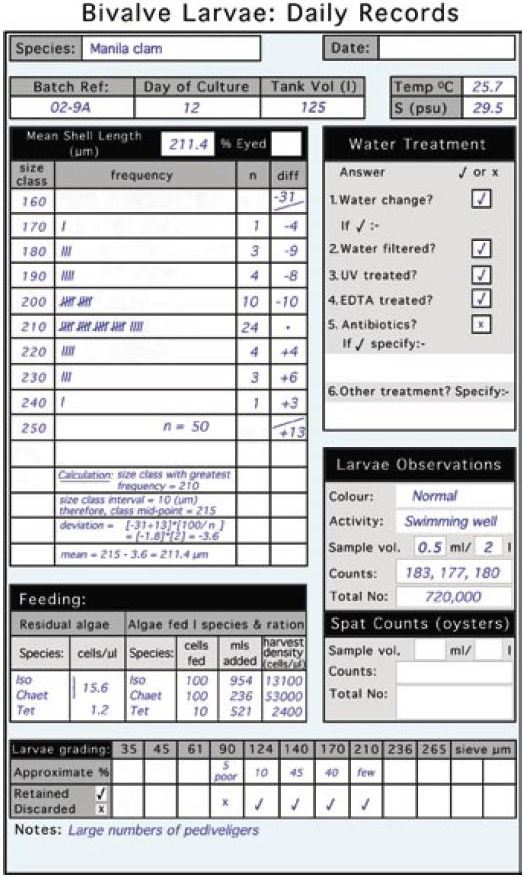
Figure 60: An example of a daily record sheet and the type of information that is useful to record in order to follow the progress of a batch or a tank of larvae. The steps in calculating the mean shell length of larvae from size/frequency plots are also shown.

Figure 61: Draining static larval tanks on water change days.
The procedure is as follows:
a) Wash any remaining larvae from the vessel into the sieve.
b) Clean the vessel with a sponge and hot detergent or bleach solution and rinse well.
c) Refill the vessel with appropriately treated seawater at the required temperature and salinity.
d) Grade the larvae by washing them through a stack of sieves of descending aperture size with filtered seawater. A guide to suitable mesh sizes for larvae of different shell lengths is given in Table 11.
e) Take small samples from each sieve upon which larvae have been retained and observe the appearance and activity of the larvae with the aid of a microscope. Discard any sieve fraction containing predominantly dead or slow-growing larvae.
Note: Sieve fractions containing mainly empty shells and larvae with decomposing tissues need to be discarded. The tissues of healthy larvae are of golden-brown colouration with a well defined and darkly coloured digestive gland. Moribund larvae tend to be more darkly and uniformly granular in appearance.
f) Wash fractions containing healthy larvae into a measuring cylinder.
g) Take sub-samples as described before and determine the total number surviving. Measure a sample of 50 to 100 and calculate mean shell length.
Note: The addition of a few drops of formalin (10% formaldehyde solution, neutralized over calcium carbonate in the form of limestone or marble chips) will immobilize the larvae. Discard the counted sample(s).
h) Return the larvae to the culture tank and restore the aeration.
i) Repeat this procedure at 48-hour intervals.