5.1.2 Methods for embryo development
5.1.2.1 Tanks for embryos and larvae
Fertilized eggs are permitted to develop to the fully-shelled “D” veliger stage in tanks of the type shown in Figures 50 and 52. This early veliger stage is known as the D-larva stage because of the characteristic “D” shape of the shell valves (Figure 51). D-larvae of the various, commercially cultured bivalves are similar in appearance.
A wide range of circular or semi square (square with rounded corners) tanks can be used for embryo development and also for larval rearing (Figure 52).
They should be constructed from preferably either “virgin” (new, non-recycled) polyethylene or fibreglass (alternatively known as GRP – glass reinforced plastic or fibreglass). Previously unused vessels should be filled with seawater and allowed to soak with weekly changes of water for 2 to 4 months before use. Soaking removes toxic substances that leach from the surface of new plastics which may be harmful to larvae.
Steam curing of fibreglass tanks substantially reduces the period the tanks need to be seawater soaked.
Flat-bottomed or steeply tapering

Figure 50: Fertilized eggs can be incubated in various types of tanks in filtered seawater for a period of 2 to 3 days, depending on species and temperature.
conical tanks (i.e. almost flat bottomed) are most commonly used for embryo development (Figure 52). Shallowly tapering
conical tanks (shaped like an ice cream cone) are less satisfactory because the early embryos are immobile and will tend to aggregate together at the bottom of the cone.
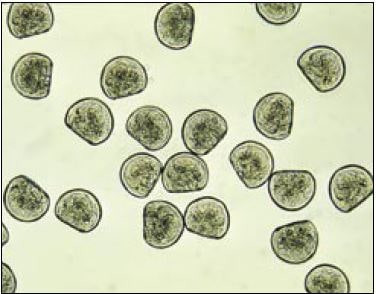
Figure 51: Photomicrograph of Crassostrea gigas D-larvae (48-h after fertilization). Mean size is 75 ?m shell length.
Surface area of the tank base rather than water depth is more important. Aeration during this early stage is not recommended. The mechanical effects of the disturbance it creates can lead to abnormal development.

Figure 52: Suitable rearing vessels for embryo (and larval) development. A – 200 l steeply tapering conical fibreglass tank with bottom drain; B – 125 l polyethylene flat-bottomed tank; C – 1 000 l insulated polyethylene, square tank with rounded corners.
5.1.2.2 Water treatment
Culture tanks are filled with seawater filtered to 1 to 2 ?m particle size (Figure 53A) and heated to the required temperature (usually 18 to 24oC; cooler for cold water species). Some hatcheries disinfect the water following fine filtration by passing it through an ultra-violet light (UV) unit (Figure 53B), the value of which is questionable unless it is used properly and with discretion.
UV units should be maintained according to manufacturer’s recommendations and a record kept of hours of lamp usage. Lamps must be replaced as they reach the specified hours of use at which time the quartz silica sheath that separates the lamp from the water flow needs to be cleaned with a soft cloth soaked in alcohol. Moreover, these units are designed to disinfect freshwater and are not as efficient in killing or immobilizing marine bacteria and other micro-organisms.
As a rule of thumb – if UV disinfection is considered necessary – it is best to pass water through two or three similar units, connected in series, at half the flow-rate recommended for a single unit (Figure 53). It should be remembered that limiting the diversity of bacteria in a culture of embryos or larvae may reduce competition and thereby permit potentially harmful bacteria to predominate. Modern thinking is that the probiotic approach is the better option. This approach entails controlling the density of larvae carefully, feeding them properly with only the best cultured algae available and, paying attention to the hygienic operation of both the cultures and equipment.
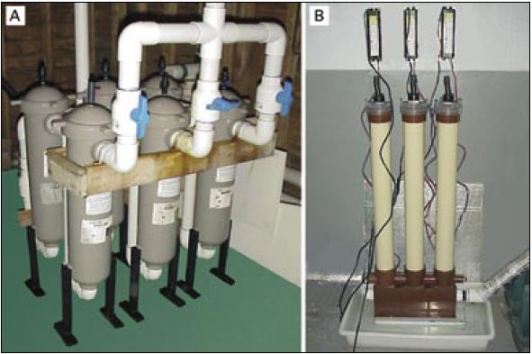
Figure 53: Examples of suitable equipment for water treatment. The multiple bag filtration unit (A) is arranged for fine water filtration. One bank of 3 filters is in use while the second bank is serviced and readied for use. These filtration units contain bags that progressively remove par ticulate matter from 10 ?m down to 2 ?m in three stages. The uv disinfection unit (B) consists of 3 lamp units arranged in series and is designed to treat a continuous flow of previously filtered seawater. This is the recommended arrangement in seawater treatment rather than relying on the water being treated by a single lamp unit.
It is sometimes beneficial to filter the water and to fill the culture tanks 24 hours before they are needed. This is more applicable in hatcheries located adjacent to estuaries contaminated by industrial or domestic wastes, or by the leachings from richly metaliferous geological strata (and mine workings) in the catchment area, which may contain elevated quantities of heavy metals. The water is then treated by adding 1 mg per l of EDTA (sodium salt – as used in the preparation of algal culture medium) and 20 mg per l of sodium metasilicate and is vigorously aerated for 24 hours. Pre-treatment helps to complex heavy metals and render them non-toxic to the particularly vulnerable early stages in development of bivalve larvae. The water does not need to be re-filtered after pre-treatment but aeration is switched-off during embryo development.
5.1.2.3 Culture of embryos
Embryos are stocked in the culture tanks about 2 hours after fertilization and at the appropriate density. Fully developed D-larvae are recovered 24 to 48 hours later, depending on species and water temperature (Figure 54). Either no or very low aeration is used during embryo development.
Embryo stocking densities for many of the commonly cultured oviparous oysters and clams can be as high as 50 000 to 80 000 per l of culture, although 20 000 per l is more generally considered to be the safe upper limit (Table 10). In contrast, similarly high initial embryo densities of many of the scallop species leads to abnormal development and numbers are usually restricted to 10 000 to 15 000 fertilized eggs per l of culture tank volume in warmer water species. Egg densities are more commonly based on the surface area of the tanks rather than on tank volume in cold water scallop species, where maximum density should not exceed 1 000 per cm2 (Table 10).
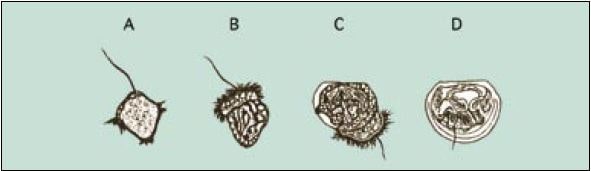
Figure 54: Development of embryos from the early trochophore (A) to the fully shelled D-larva stage (D). The ciliated swimming feeding organ (velum) can be seen in B and early shell valve formation in C. Fertilized eggs will develop to fully formed D-larvae in less than 2 days in many warm water species but the entire developmental process can take 4 or more days in cold water species.
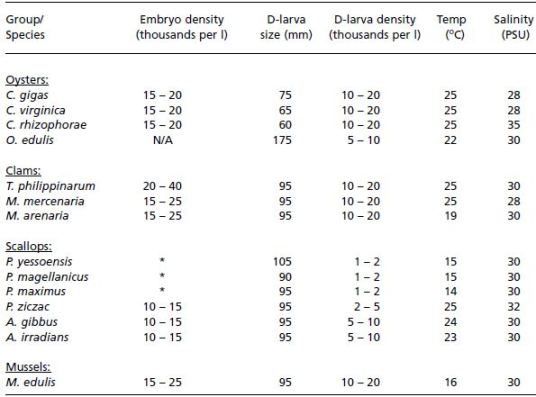
Table 10: Summary data of typical embryo densities (thousands per l), initial D-larva size (shell length, ?m), densities of D-larvae (thousands per ml) and culture conditions in terms of suitable temperature (+ 2oC) and salinity (+ 5 PSU) for the culture of embryos and early larvae of a number of bivalves. Notes: N/A – not applicable: embryo development takes place within the mantle cavity in Ostrea edulis. * Embryo densities in cold water scallops are calculated as embryos per unit area of the base of tanks rather than per unit volume. Maximum density should not exceed 1 000 fertilized eggs/embryos per cm2.
A recovery of from 30% to 85% perfectly formed D-larvae from the initial number of embryos stocked is normal in large-scale rearing. Imperfectly formed D-larvae – those with incomplete or misshapen shells – rarely develop further.
Fully shelled D-larvae have a mean shell length of 90 to 100 ?m in most species of clams, scallops and mussels, and 55 to 75 ?m in oviparous oysters of the genus Crassostrea (Table 10). Crassostrea gigas has larger D-larvae than either Crassostrea virginica or Crassostrea rhizophorae.
A special case is the larviparous oysters of the genera Ostrea and Tiostrea, which have considerably larger eggs. They brood larvae until they are released into the surrounding water at 170 to 200 ?m shell length (retained by a 90 ?m mesh screen) in the case of Ostrea edulis and an average length of 490 ?m in Tiostrea species (see part 4.2.3). Tiostrea
larvae are liberated at the pediveliger stage (the pre-settlement and metamorphosis stage) and are ready to set almost instantaneously (<1 hour after release).
Shell length is best measured with a monocular microscope (x100 magnification) fitted with an eye-piece graticule calibrated against a micrometer slide (Figure 55).
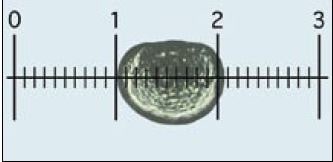
Figure 55: Measuring larvae: each larva is oriented and lined-up with the calibrated eye-piece graticule, as shown, and the number of small sub-divisions it spans on the scale, equivalent to shell length, is recorded. In this case, at x100 overall magnification (x10 eye-piece and x10 objective), each small division is 10 µm. Thus, the D-larva shown measures approximately 105 µm.
Normal D-larvae are retained by a 45 ?m nylon mesh-based sieve (35 ?m in the case of D-larvae of Crassostrea gigas, or 25 ?m for C. rhizophorae and C. virginica) and the number recovered is estimated as described later in section 5.1.2.3.
Recovering D-larvae
Tanks containing newly developed D-larvae are drained 2 days after fertilization. There is merit in adding a small quantity of food to the tank on the day prior to draining, i.e. 24-h to 36-h after fertilization. While embryos develop to the D-larvae stage utilizing maternally derived reserves, fully developed D-stage larvae are able to ingest algal cells of the smaller food species and benefit from uptake of dissolved nutrients.

Figure 56: The arrangement of sieves to capture D-larvae from a tank where a smaller diameter, 60 ?m mesh sieve is suspended over a larger diameter 40 ?m sieve which is partially immersed in a shallow tray containing the discharge seawater.
This arrangement permits grading of larvae by size at the point of collection and ensures larvae are not allowed to dry out.
The method employed to catch and retain larvae while tanks are being drained is illustrated in Figure 56. When the tank is full, the drain valve is opened a fraction to allow a slow flow of water into a sieve, or series of sieves, contained in a shallow tray. This arrangement ensures the mesh of the bottom sieve is always immersed in seawater, which minimizes damage to the shells of the fragile D-larvae during draining. As the tank drains down, the valve can be opened further making certain that the flow is not sufficiently violent so as to cause excessive turbulence. Any larvae retained in the tank once it is empty are flushed out with a flow of filtered seawater. Tanks without drains can be emptied by siphoning the water through a similar arrangement of sieves using a length of flexible hose.
D-larvae can be graded during tank emptying by suspending a slightly larger aperture sieve over one with a smaller mesh aperture, as in Figure 56. This is often of benefit and can help separate the better, larger D-larvae from those that are imperfectly formed and abnormal (Figure 57). Once the tank has been completely emptied, filtered seawater is gently sprayed over larvae retained in the upper sieve to wash any smaller individuals into the lower sieve. The contents of larvae in both sieves are washed into separate, graduated containers and are then estimated for numbers and examined as described below. At the same time small sub-samples are often taken to be subsequently measured for shell length.

Figure 57: The appearance of almost 5 million calico scallop, Argopecten gibbus, larvae concentrated in a 20 cm diameter sieve (A) and after transferring to a 4 l graduated jug, preparatory to estimation (B).
Estimating Egg, Embryo and Larval Numbers
Care needs to be taken in handling eggs and larvae. When transferring eggs, embryos or larvae from one container to another through a mesh-based sieve, always ensure that the mesh of the sieve is submerged below the surface of the receiving container. All equipment used in transfers of this kind and in the estimation of numbers should be thoroughly cleaned beforehand and rinsed with filtered seawater.
(i) equipment required
Much of the equipment used for the purpose of estimating larval numbers needs to be specially made. For example, sieves are prepared from PVC pipe or high-impact, rigid styrene garden plant pots or horticultural containers. (Proprietary screens/sieves constructed of metal should be avoided).
To make suitable sieves, the bases of plastic containers are removed and nylon monofilament mesh is fixed tautly in place at the cut end with suitable solvent cement. Alternatively, 15 cm sections of suitable diameter PVC pipe (20 to 30 cm diameter is convenient) are cut and nylon mono-filament mesh fitted to one end in the same way. Sieves should be indelibly marked with mesh size for easy identification.
It is useful to make a number of sieves for each of a wide range of mesh sizes ranging from 20 ?m to 250 ?m for the various purposes of embryo, larvae and early juvenile culture. Useful sieve sets for clam and scallop larvae are 40, 60, 80, 120 and150 ?m mesh aperture (Table 11). The range needs to be extended upwards for larvae of the various commonly cultured oyster species.
Perforated plunger agitators and counting slides can be made in the workshop from plexiglass or transparent PVC pipe, sheet, and rod. Proprietary sedgewick rafter slides, available from scientific and aquaculture suppliers are useful for counting purposes (Figure 58).
Table 11: The relationship between the mesh aperture of sieves (screens) and the minimum size of larvae they will retain. This information is for guidance only and differs from species to species according to the shape of larvae. Experienced hatchery technicians can estimate the mean size of larvae from a culture by their distribution and retention on a range of mesh sizes at grading.
Figure 58: Equipment used in estimating numbers of larvae. A – perforated plungers for evenly suspending larvae in containers from which known volume sub-samples are taken in the estimation of larval numbers. B – a sedgewick rafter microscope slide, which has a chamber designed to take a 1 ml sample. The chamber has a grid marked on the base for easy tracking while viewing and counting larvae or spat in the sample. Similar slides can be made from transparent plastic.
Specific equipment that needs to be purchased includes adjustable volume automatic pipettes (0.1 to 1.0 ml and 1.0 to 5.0 ml ranges are useful); various measuring cylinders from 25 ml to 2 l volume and wash bottles.
Where budget permits, an electronic particle counter performs the same task as described below and saves time. It is also extremely useful for counting cell density in algal cultures and in determining food cell consumption at all stages in the hatchery process (refer back to Figure 21).

Figure 59: The steps in taking sub-samples of larvae for counting in the estimation of total numbers. A – taking a sub-sample with an automatic pipette while agitating the contents of the jug containing the pool of larvae; B – transferring the sub-sample to a sedgewick rafter slide; C – counting and recording the number of larvae in the sub-sample. The lower photographs (D and E) show a similar technique where larvae, concentrated in a 2 l graduated cylinder are sampled by automatic pipette during agitation.
(ii) estimating procedure (Figure 59)
a) After sieving and rinsing eggs, newly fertilized embryos or larvae, transfer them to a graduated measuring cylinder (1 or 2 l volume) or, if numbers are anticipated to exceed 5 to 10 million, to a bucket or similar larger volume container graduated in l, pints or gallons.
Note: For greater accuracy when using larger volume containers, use an accurately calibrated measuring cylinder or jug to fill the container to about 8 cm below the rim. Note the volume added and mark a calibration line on the inside of the container at the waterline with an indelible marker.
b) Add filtered seawater to the container to the graduation mark. (The total volume needs to be known).
c) With an automatic pipette set at 0.5 ml (volume may vary – see later) take 3 replicate sub-samples of the contents while agitating the contents of the measuring cylinder, or other container, with a suitable diameter perforated plunger. Make sure that the eggs or larvae are evenly distributed in the water column during sub-sampling (Figure 59A).
Note: The diameter of the plunger should be slightly, but not considerably, smaller than the diameter of the container to be sampled. Agitation should be sufficient to lift eggs or larvae off the bottom of the container and evenly suspend them, but not vigorous enough to cause excessive turbulence. A slow, rhythmical up and down motion at about 1 complete cycle per 4 seconds is recommended.
d) Transfer the sub-samples to the compartments of the counting slide (Figure 59B). The compartments should be etched with a suitable grid, as illustrated in.
Note: Take smaller volume sub-samples when numbers of larvae are anticipated to be large, or use a larger volume measuring cylinder/graduated container, or a combination of the two. In the case of eggs, which are very delicate, it may be easier to transfer the egg suspension to a large container, for example, a 10 l polyethylene bucket. Make up the volume to the calibration line and withdraw sub-samples while gently agitating the bucket contents with a large diameter, perforated plastic plunger.
e) Count the eggs or larvae in each sub-sample using a microscope (x40 magnification – Figure 59C).
Note: In the case of eggs and newly fertilized embryos, separate counts can be made of the total number per sub-sample and the number that have not rounded-off and appear abnormal. The same procedure applies to D-larvae where calculation can be made, based on the counts, of the percentage that have developed normally. Similarly, mortality rate estimation can be made on later larvae as part of the counting procedure by counting the live and dead or moribund larvae separately.
f) Calculate the total number as in the following example:
Example:
Larvae counts in the three sub-samples = 414; 389; 402.
Mean = 414 + 389 + 402/3 = 402
Volume of sub-sample = 0.5 ml.
Total volume of cylinder = 2 000 ml.
Total number of larvae = 2 000/0.5 x 402 = 1 608 000
Eggs and larvae can also be counted using an electronic particle counter (e.g. Coulter Counter) fitted with a suitable aperture, sampling head. While this method is quick and convenient, it is impossible to distinguish between normally and abnormally developing larvae or between dead and live larvae. There is no substitute for visual examination and discrimination of the quality of a culture by an experienced hatchery technician.