3.2. Characteristics
Duckweeds are adapted to a wide variety of geographic and climatic zones and are distributed throughout the world except in regions where temperature drops below 0 ?C during part of the year. Most species are found in moderate climates of tropical and temperate zones. In deserts and extremely wet areas, duckweeds are rare.
Lemna spp., for example are very rare in regions with high or very low precipitation and are not found in Greenland or the Aleutian Islands (Landolt, 2006). Although many species can survive extremes of temperature, they generally grow faster under warm and sunny conditions (Skillicorn, Spira and Journey, 1993). Most species show prolific growth in the tropics. Various microclimatic factors such as light intensity, salinity, regional temperature differences can influence the distribution of Lemnaceae species (Landolt, 1986). Birds and floods often disperse duckweeds to different geographic areas.
3.2.1 Reproduction
A duckweed plant consists of a single leaf or frond with one or more roots. Most species of duckweed multiply principally through vegetative propagation by the formation of daughter fronds from two pockets on each side of the narrow end of the frond (Gaigher and Short, 1986). Newly formed fronds remain attached to the mother frond during the initial growth phase and the plants therefore appear to consist of several fronds. Species of the genus Spirodela have the largest fronds, measuring as much as 20 mm across, while those Wolffia species are 2 mm or less in diameter. Lemna species are intermediate in size, being about 6-8 mm.
An individual frond may produce as many as 20 daughter fronds during its lifetime, which lasts for a period of 10 days to several weeks. The daughter frond repeats the history of its mother frond. Some of the duckweed species, however, reproduce by producing unisexual and monoecious flowers and seeds. For example, L. paucicostata routinely flowers and seeds. However, the flowers are very small and rare in many species; male and female flowers are borne on the same plant. Each inflorescence generally consists of two male flowers and one female, but in Wolffia, there is one male and one female. The flowers are naked or surrounded by spathe. The fruit is a utricle and the seeds are smooth or ribbed. Vegetative reproduction is very rapid and is usually by the formation of buds of new fronds from the reproductive pouches (Guha, 1997).
Many species of duckweed survive at low temperatures by forming a special starchy ‘survival’ frond known as a turion. In cold weather, the turion is formed and sunk to the bottom of the pond where it remains dormant until warm water triggers resumption of normal growth. Several species survive at low temperatures without forming turions. During the winter season, the fronds are greatly reduced but remain at the surface. Occasionally, turion-like fronds will form, but the plants continue to slowly reproduce vegetatively. These plants are probably the best plants to utilize in a culture system, as restocking is virtually assured. L. gibba, L. valdiviana, L. minor, L. trisulca and L. minuscula are five duckweed species that frequently show some growth at cold temperatures.
3.2.2 Environmental requirements
A variety of environmental factors, such as water temperature, pH and nutrient concentration, control the growth and survivability of duckweeds. The other environmental factors that influence the growth rates of duckweed colonies are presence of toxins in the water, crowding by overgrowth of the colony and competition from other plants for light and nutrients. However, the growth rate of duckweed is favoured by organic pollutants as well as inorganic nutrients (Guha, 1997). The effect of these various factors is summarised below.
Temperature
Temperature tolerance and optima are dependent on species and possibly even on clones. Optimum temperature for maximum growth of most groups apparently lies between 17.5 and 30 ?C (Culley et al., 1981; Gaigher and Short, 1986). Although some species can tolerate near freezing temperatures, growth rate declines at low temperature. Below 17 ?C some duckweeds show a decreasing rate of growth (Culley et al., 1981). Most species seem to die if the water temperature rises above 35 ?C. The effect of temperature on growth is affected by light intensity, i.e. as light increases, growth rates increase from 10 to 30 ?C.
In Bangladesh, Khondker, Islam and Nahar (1993a) reported the temperature dependent growth of S. polyrrhiza with a maximum biomass of 76.4 g/m2 recorded in the middle of February, after which the biomass depleted gradually with the rise in water temperature. The water temperature in the middle of February was about 19 ?C. Islam and Khondker (1991) also obtained a high growth of S. polyrrhiza at a temperature of 16 ?C. Furthermore, Khondker, Islam and Nahar (1993b) reported maximum growth of S. polyrrhiza at water temperatures of 22.2-22.5 ?C in a growth study conducted in pond water. Khondker, Islam and Makhnun (1994) reported an inverse correlation between water temperature and the biomass of L. perpusilla when the water temperature varied between 15 and 28 ?C. These authors also noted that the growth of this duckweed species ceased completely at 26 ?C and above.
pH
Duckweeds are generally considered to have a wide range of tolerance for pH. They survive well from pH 5 to 9, although some authors put their range between 3 and 10. However, pH tolerance limits of the various species differ. Stephenson et al. (1980) noted that duckweed display optimum growth in a medium of pH 5.0-7.0. Generally, duckweeds grow best over the pH 6.5 to 7.5 range. A doubling of biomass in 2 to 4 days has been demonstrated at pH levels between 7 and 8 (Culley et al., 1981). Unionized ammonia is the preferred nitrogen substrate for duckweed. An alkaline pH shifts the ammonium-ammonia balance toward the un-ionized state and results in the liberation of free ammonia, which is toxic to duckweed at high concentrations (100 mg NH3/L).
Islam and Paul (1977) observed that W. arrhiza grew at a pH range of 5-10, although the optimum pH was found to be 7-8. In Bangladesh, S. polyrrhiza has been reported to grow best at a pH between 6.5 and 7.5 (Islam and Khondker, 1991). The range of pH for optimum growth of S. polyrrhiza reported in India was 6.8-8.5 (Kaul and Bakaya, 1976; Gopal and Chamanlal, 1991). Khondker, Islam and Makhnun (1994) reported the pH range of 6.9 and 7.8 to be suitable for the growth of L. perpusilla. Similarly, Van der Does and Klink (1991) observed pH of 7.36 in a lemnid habitat in the Netherlands supporting growth of L. perpusilla. A summary of minimum, maximum and optimum pH of various duckweed species is presented in Table 3.2.
Conductivity
Electrolyte conductivity appears to have some effect on the growth of different species of duckweed. Zutshi and Vass (1973) found L. gibba and L. minor growing in stagnant waters rich in electrolyte ranging from 400-500 µS/cm. Gopal and Chamanlal (1991) reported the maximum biomass of L. perpusilla and S. polyrrhiza from roadside pools and ditches in India within a electrolyte conductivity range of 650-1 000 µS/cm. Khondker, Islam and Nahar (1993a) recorded the complete disappearance of
Table 3.2
Minimum, maximum and optimum pH of various duckweed species
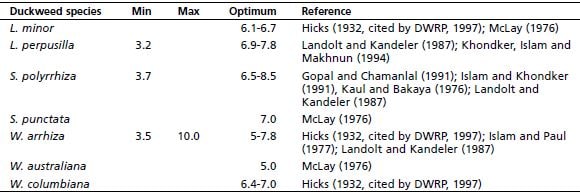
Source: DWRP (1998)
S. polyrrhiza by the end of May when a sharp fall in conductivity and alkalinity was observed. The electrolyte conductivity of water supporting the growth of L. perpusilla in Bangladesh reported by Islam and Khondker (1991) and Khondker, Islam and Makhnun (1994) were 625 µS/cm and 200-890 µS/cm, respectively. High electrolyte conductivity (1 090 µS/cm) of water supporting the growth of L. perpusilla was also reported by Van der Does and Klink (1991) in a lemnid habitat in the Netherlands.
Nitrogen
In general, temperature and sunlight control duckweed growth more than nutrient concentrations in the water. At high temperatures, duckweed can grow rapidly down to trace levels of phosphorus and nitrogen. The crude protein content of duckweed however, seems to increase to a maximum of ~40 percent DM over the range from trace ammonia concentrations to 7-12 mg N/L (Leng, Stambolie and Bell, 1995). Culley et al. (1981) reported that the TKN of water should not drop below 20-30 mg/l if the optimum production and a high crude protein content of duckweed are to be maintained.
Duckweeds prefer ammonia nitrogen (NH4-N) as a source of nitrogen and will remove ammonia preferentially, even in the presence of relatively high nitrate concentrations. Luond (1980) demonstrated that higher growth rates were attained when nitrogen was in the NH4-N rather than the NO3-N form. In organically enriched waters, nitrogen tends to be concentrated in the NH4-N rather than the NO3-N form at pH levels below 9 and plant growth is equally efficient in anaerobic and aerobic waters (Said et al., 1979). In lagoons receiving organic animal wastes, the pH seldom exceeds 8, particularly with a full duckweed cover that suppresses phytoplankton growth (Culley et al., 1978). The plants can tolerate very high ionized ammonia (NH4-N) concentrations but the effects of unionized ammonia (NH3-N) have not been clearly demonstrated. Urea is a suitable fertilizer and is rapidly converted to ammonia under normal conditions. According to the results of laboratory experiments, duckweed tolerates concentrations of elemental N as high as 375 mg/l (Rejmankova, 1979).
Phosphorus and potassium
Phosphorus is essential for rapid growth and is a major limiting nutrient after nitrogen, although its quantitative requirement for maximum growth is generally low. Fast growing duckweed in nutrient rich water is a highly efficient sink for both phosphorus and potassium; little of each, however, is required for rapid growth. Saturation of phosphate uptake by duckweed occurs at available PO4-P concentrations of 4 to 8 mg/l. Duckweed growth is not particularly sensitive to potassium or phosphorus once an adequate threshold has been reached. Rejmankova (1979) reported good growth of duckweed within the P concentrations of 6 to 154 mg/l. Culley et al. (1978),
34 Use of algae and aquatic macrophytes as feed in small-scale aquaculture – A review
working in dairy waste lagoons, achieved doubled production from 2 to 4 days at P concentrations in excess of 35 mg/l. Reduced growth in some species occurs only after P values dropped below 0.017 mg/l (Luond, 1980). Khondker, Islam and Makhnun (1994) observed that both phosphate and silicate concentrations had significant positive correlation with the biomass of L. perpusilla in Bangladesh.
Other minerals
A range of other important mineral levels found in water supporting Lemnaceae is presented in Table 3.3. Although these minerals are essential for their survival, duckweed growth is not particularly sensitive to potassium or phosphorus once an adequate threshold has been reached.
Table 3.3
Range of important mineral contents (mg/l) of water supporting Lemnaceae
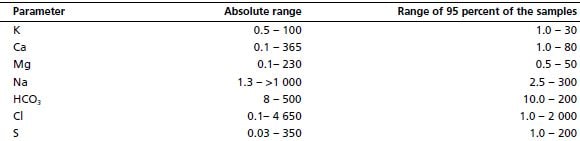
Source: modified from Landolt (1986)
In summary
Maximum, minimum and optimum requirements of some of the most important environmental parameters (temperature, pH, conductivity, nitrogen and phosphorus) are given in Table 3.4. It is apparent that the duckweeds are robust in terms of survival, but sensitive in terms of thriving. They have extreme range of tolerance for temperature, pH, conductivity, nitrogen and phosphorus with well-defined range of optimum requirement.