2.2 SCIENTIFIC BACKGROUND – LIVE ALGAE AS FOOD
The algal culture facility is a most important part of a bivalve hatchery. Adequate quantities of high quality food must be available at all times for successful operation, and a failure in the algal culture facility can be catastrophic to the hatchery. Larval and adult scallops feed on unicellular phytoplankton. Although studies have shown that it is possible to grow bivalve juveniles on a non-algal diet (Langdon and Siegfrid, 1984; Chu et al. 1987), the manufacture of artificial diets for bivalve hatcheries is still in the experimental stage.
Micro-encapsulated diets (Langdon and Onal, 1999; Davis and Campbell, 1998) have been only partially successful in replacing live unicellular algae, which remain the major source of nutrition for filter-feeding organisms. In a compact
Technical drawing, Pg. 12
Algal culture unit: Details & photos
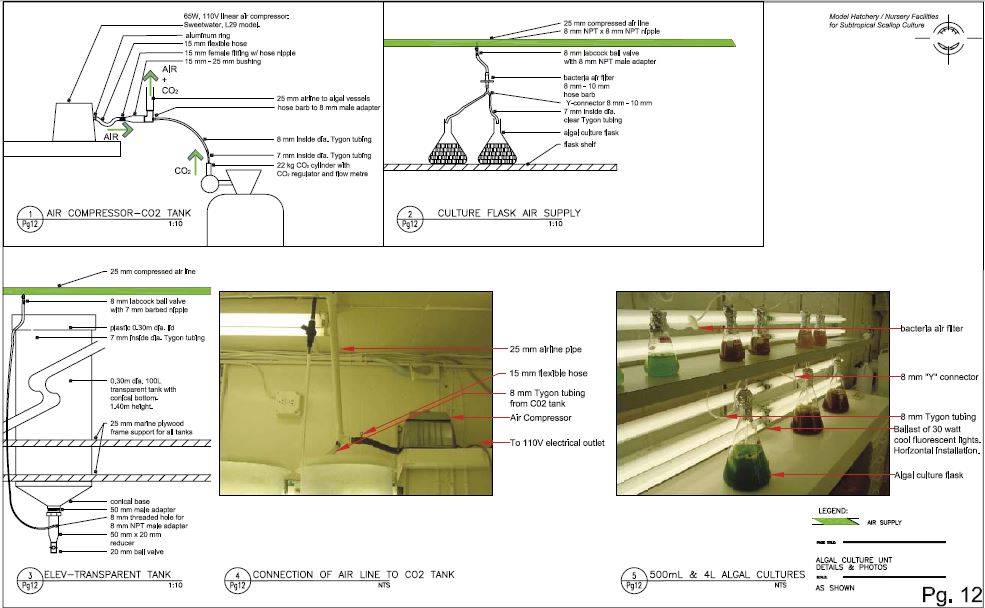
and space-restricted facility such as the one described here, the heaviest load for algal culture production is the broodstock and older spat requirements. For this reason, dry algae are purchased from Reed Mariculture, for the feeding of broodstock and 2–5 mm spat at the BBSR facility. Dry algal use is discussed in Section 2.3.
Live algal culture techniques used at BBSR are described below.
2.2.1 Algal growth and composition
Growth of unicellular algae is by simple cell division, i.e. a single cell divides to form two cells, which then divide to form four cells, etc. Under normal conditions, an algal culture goes through three phases of growth: lag, exponential and stationary phases (Figure 2.1). The lag phase occurs when the culture is started and little increase in cell density is observed. In healthy cultures this period is quite short. In the exponential phase cell division occurs rapidly and cell density increases geometrically. Growth is limited only by the time required for cell division in this phase. In the stationary phase, the rate of growth (cell division) declines because some factor, such as nutrients or light, has become limiting and cell density remains relatively constant. During these different phases of growth the biochemical content of the algae differs. It has been shown that higher energy levels are found in the
stationary phase for most species, the diatom Thalassiosira being an exception (Whyte, 1987). In a hatchery situation, a balance between high cell density and optimal energy content is strived for. For this reason, the strategy at the Bermuda hatchery is to maintain cultures in the exponential phase of growth, thus remaining healthy and continuously dividing, and harvest them at the beginning of the stationary phase, when energy content peaks. Cultures are harvested within 3 days, thus remaining for a short time in the stationary phase, and preventing the presence of a large number of dying cells within the culture.
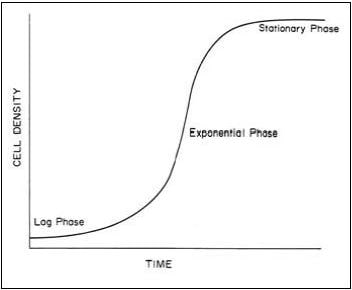
Figure 2.1: Theoretical growth curve of typical algal culture showing lag, exponential and stationary phase (taken from Bourne, Hodgson and Whyte, 1989).
2.2.1.1 Selecting algal species
Unicellular marine algae are widely used as food in the hatchery production of commercially valuable fish and shellfish. Of the many species of algae occurring in the world's oceans, only a handful are routinely used for their nutritive quality in hatcheries. Their success as food species depends not only on their nutritional quality, but also on their tolerance to temperature, salinity and light. Because of this, algal species will exhibit different growth performance depending on the hatchery site. Knowledge of the optimal growth responses of microalgae under local conditions is of great benefit, but a first selection can be based on the literature, as much has been reported on biochemical constituency of microalgae with respect to environmental factors (Brown et al., 1997; Volkman et al., 1989; Brown et al., 1993; Whyte, 1987). The first criteria, in selecting a range of microalgal species is thus dependent on the environmental conditions of the hatchery site itself.
Culture conditions such as, nutrient media used, temperature, light, quality of seawater, and the phase of growth (Brown, 1991; Moal et al., 1987; Wikfors, Twarog and Ukeles, 1984; Dortch, 1982; Fabregas et al., 1986), affect the total concentrations of protein, lipid and carbohydrate in microalgae. Selecting the right algal species is of importance, as the performance of larvae and juveniles in terms of growth and survival is dependent on the content and nature of biochemical constituents in the algal food.
Despite variations among species, protein is usually the major organic constituent, followed by lipid and then by carbohydrate. Whyte, Bourne and Hodgson (1990) stress the importance of carbohydrate in providing a balanced diet for effective conversion of dietary macronutrients to tissue and energy reserves; according to these authors nutritional condition of the larvae correlated in their study with the content of dietary carbohydrate rather than dietary lipid or protein. Microalgal species used in bivalve culture can be divided into two groups: diatoms and flagellates. Whyte (1987) found that Chaetoceros sp. and Thalassiosira diatoms had a reduced organic content compared to flagellates, such as Isochrysis and Tetraselmis; on the other hand, diatoms in general do contain higher levels of carbohydrates than flagellates. Considering the total caloric content, Whyte (1987) found that Isochrysis species were major sources of energy followed by Chaetoceros calcitrans, Tetraselmis suecica, Thalassiosira pseudonana and finally Chaetoceros sp. All of these species have been shown to promote excellent growth for larval and juvenile oysters (Enright et al. 1986) and scallops (Bourne, Hodgson and Whyte, 1989). The second main criteria in selecting algal species, is based on the species of bivalve cultured and its specific requirements. Nutritional requirements for bivalves not only vary among species, but also among the various life cycle stages (larval, post-larval, juveniles and adults); for example, size of an algal cell, presence of spines, or cell wall thickness may preclude use of an algal species as larval food. Extensive research has been conducted for some bivalve species (Davis and Guillard, 1958; Epifanio, Valenti and Turk, 1981; Wikfors, Twarog and Ukeles, 1984). Bourne, Hodgson and Whyte (1989) give a detailed account for each life stage for the Japanese scallop, Pactinopecten yessoensis. These authors note that young larvae may not be capable of ingesting some of the longer chained algal species, such as Skeletonema costatum, or larger algae such as T. suecica; the siliceous spines of some Chaetoceros species (such as Chaetoceros gracilis), have also been shown to be unsuitable for younger larvae. However, Chaetoceros sp. are used in larger larvae, as well as juveniles and broodstock; in fact, the biochemical composition of C. gracilis, makes it the best single algal diet for conditioning broodstock and juveniles.
Generally, in order to obtain optimum performance in bivalve culture at any stage, a mixed diet of two or three algal species, is found necessary to provide all of the essential constituents. Protein is required for tissue production, carbohydrate for metabolism, and fatty acids for lipid storage and metabolic requirements. The type of fatty acid is also an important consideration, where the polyunsaturated fatty acids (PUFAs) 20:5w3 and 22:6w3 produce the best growth for juvenile oyster and scallops (Enright et al. 1986; Whyte, Bourne and Hodgson, 1989). More specifically, Bourne, Hodgson and Whyte (1989) conclude that for complete complements of fatty acids for larval scallops, a mixed diet of any of the diatoms with Tahitian Isochrysis is optimal. It therefore becomes clear that algae with more balanced proportions of micronutrients should be of higher food value to bivalves, assuming other nutritional factors are comparable such as, cell size, digestibility, and freedom from toxins or growth inhibitory metabolites.
Species listed in Table 2.1 are commonly used in bivalve hatcheries worldwide and are recognized as suitable species in terms of ease of culture and nutritive value. Coutteau and Sorgeloos (1992) found Tahitian Isochrysis used in 72 percent of hatcheries, while only 37 percent of hatcheries reported using C. calcitrans. Despite the favourable results in several studies (O’Connor and Heasman, 1997; Peirson, 1983; Nell and O’Connor, 1991), Pavlova lutheri was used by only a quarter of hatcheries surveyed (Coutteau and Sorgeloos, 1992). Observations have shown a limited tolerance of this species to temperatures above 27 °C, which may explain its limited use, especially in the tropics.
Table 2.1: Commonly used species of micro algae in bivalve hatcheries.
Algae Cell diameter
(µm)
Diatoms
Skeletonema costatum 6
Chaetoceros calcitrans 2.5 Chaetoceros gracilis 6
Phaeodactylum tricornutum 5
Thalassiosira pseudonana 5.5 Chaetoceros muelleri 3
Flagellates
Tetraselmis suecica 8.5 Isochrysis galbana or T-Iso 5
Nannochloris occulata 2
Dunaliella tertiolecta 6.5 Pavlova lutheri 5
Tetraselmis chuii 8.5
The algal species cultured at the BBSR hatchery over the course of four years for the rearing of Euvola ziczac and Argopecten gibbus are: Tahitian Isochrysis, Isochrysis sp., C. calcitrans, C. gracilis, Chaetoceros muelleri, T. pseudonana (clone: 3H), T. suecica and Tetraselmis chuii. Several studies were conducted at the Bermuda hatchery, evaluating growth performance of algae. From these in-house trials, it was found that Isochrysis sp., C. muelleri and T. chuii were best suited to Bermuda’s culture conditions.
Furthermore, these species yield satisfactory growth rates for larval and post-larval stages. Due to space limitations in Bermuda, it is found more manageable to culture a few species, rather than overextend the algal culture facility by rearing a larger number of algal cultures.
2.2.1.2 Requirements for algal cultures
Several factors are necessary for algal growth. As with all plants, algae must have sufficient nutrients to support growth. For rearing high concentrations of algae, as in hatcheries, type and amount of nutrients added is crucial. There are several types of nutrient media which may be used; they can either be made using purchased chemicals, or can be purchased ready-made from aquaculture companies. The Culture Centre for Marine Phytoplankton (CCMP) website provides recipes for various culture media (F/2, L1); the Conway medium recipe is provided in Appendix 9 and was initially used at the BBSR hatchery. It is no longer used as the preparation is lengthy, although it does support excellent growth for bivalves (Sarkis, 1987). To minimize the work load, commercially available media is purchased by the BBSR hatchery; F/2 solution is easily obtained from aquaculture suppliers; supplement solutions of vitamins and sodium metasilicate are made at the hatchery, Details for the preparation of all culture media can be found in Appendix 9. Following preparation, culture media is autoclaved when used for smaller volume inoculations. Tris buffer may be added to prevent the precipitation of some chemicals during autoclaving. It also buffers the pH of the culture media during algal growth. Note: With the F/2 purchased by BBSR, the addition of Tris buffer is not found critical.
Factors, such as light, temperature, salinity, seawater quality, mixing and cleanliness are essential to algal growth. Attempting to achieve as constant culture conditions as possible will favour optimal algal growth, which will furthermore affect their biochemical composition. As mentioned previously, this is an important consideration in growth and survival of bivalve larvae and juveniles. Ensuring that harvest of algae is conducted at the same phase of growth, is a second constant to be achieved during routine culture procedure. Some of the more important criteria for successful algal growth are reviewed below, but more detailed accounts can be found in the literature.
Light is normally provided by fluorescent lamps. The most commonly used are “cool white”, but little difference has been reported when using others such as gro-lux or full spectrum (Bourne, Hodgson and Whyte, 1989). Increasing the light intensity usually means better growth and faster division of algal cells. Lamps also generate heat, and hence climate control of the algal culture housing facility is important. Most types of algae grow well between 17–22 °C. Tropical species are chosen at the Bermuda hatchery, as climate control is most easily achieved at 23–25 °C. Above 27 °C, most types of algae will die. Lower temperatures will reduce the growth rate. Ambient salinity is used at the BBSR hatchery (36 ppt), which is relatively high; nonetheless, all algal species, except for diatoms, which are reared at 25 ppt, fare well. Generally salinities between 25–30 ppt are best for the cultures of flagellates, and between 20–25 ppt for the culture of diatoms. Lower salinities can be obtained by diluting seawater with tap water or Q-water (de ionized water). Salinity can be measured with a hydrometer or refractometer. Seawater used must be clean of unwanted types of algae and other contaminants which may feed or compete with the algae. For this reason, seawater is filtered finely and sterilized or pasteurized. There is a wide range of suitable equipment commercially available for this purpose. Chemical sterilization can be easily achieved for large volumes, if no equipment is available (see Appendix 10). Carbon dioxide is provided to algal cultures for faster growth and maintenance of high densities. Carbon dioxide is supplied from compressed gas cylinders; very little is required (0.5–5 percent) in the air supplied to the culture. The CO2 should be passed through a flowmeter to facilitate the monitoring of delivery levels; the aim is to maintain a constant pH of 7.5–8.5 in all algal cultures, as algae divide and use up CO2 in the culture water. The pH can be checked with indicator papers or a pH meter if available. Both the air and the CO2 should be filtered through an in-line filter unit of 03–0.5 µm before entering the culture, as this helps to prevent other, possibly contaminating, organisms from getting into the cultures. Finally, mixing the cultures using air supply, allows all cells to be exposed to light and nutrients. Air can be supplied from a compressor or an air blower, and acts as a carrier gas for carbon dioxide. Not all aquaculturists keep smaller volume cultures mixed by bubbling. However, at the Bermuda hatchery, it is found that best results are obtained with mixing.