3.3 SCIENTIFIC BACKGROUND – FACTORS INFLUENCING LARVAL REARING
Larvae can be grown in conical or flat-bottomed vessels. Both types have been tried for rearing E. ziczac and A. gibbus. Differences in yield between conical and flat-bottomed were not truly tested, but were not apparent. Considering the restriction in space of the model hatchery, it is found that maximal tank capacity is best achieved with flat bottomed tanks.
Square “BONAR” tanks (1 000 litres capacity) are preferred as the main larval tanks mainly due to their insulation characteristic, allowing for rearing of larvae at a temperature higher than ambient. Temperature, salinity, food ration and rearing density are important factors in development of larvae. They are discussed in the following sections in relation to procedures used in Bermuda.
3.3.1 Temperature
For incubation of fertilized eggs, and larval rearing of both the calico and sand scallop, seawater temperature is increased by 6–8 °C above the ambient; such that rearing temperature is maintained at 24±1 °C for embryonic development to settlement stage. This yields an average larval life of 13 days, when pediveligers are ready for settlement in nursery systems. Costello et al. (1973) reared A. gibbus under similar conditions (T= 23±2 °C and S= 35 ppt). Velez and Freites (1993) have also reported successful culture of E. ziczac larvae at a salinity of 37 ppt and a temperature of 26 °C, yielding pediveligers within 10–12 days after fertilization.
In their review Cragg and Crisp (1991) found that time to metamorphosis in pectinids is related to temperature. Optimum embryonic and larval development varies with temperature and salinity, dependent on the scallop species and on a specific site. For example, Yamamoto (1968) and Bourne, Hodgson and Whyte (1989) report slight variations in the range of optimal temperatures for P. yessoensis (T= 10–15 °C and S= 30–40 ppt for the former, and 15–18 °C and 29 ppt for the latter). For the same species, Maru (1985) showed that optimum development of embryos occurred at a higher temperature of 20 °C. However, this does not imply that larval development will be optimal at the same temperature, and consideration to size-specific survival rate must also be given. This was shown for Pecten fumatus by Heasman, O’Connor and Frazer (1996). Embryos of this species were seen to develop best at the lowest temperatures (15–18 °C), whereas larvae initially grew rapidly at 24 °C, but did not survive to metamorphosis; a constant temperature of 21 °C was thus found to yield a maximum number of larvae for settlement. Generally, bivalve larval growth increases with temperature, up to some optimum level, which is species dependent (Bayne, 1983). However, a further increase in temperature causes growth to decline. This was shown for Chlamys hastata, where larval growth was faster at 16 °C (5.8 µm/day) than at 12 °C (4.8 µm/day), but was much slower at 19 °C (2.5 µm/day). Similarly, larvae reached a mature stage more quickly when reared at 16 °C, as compared to 12 °C (Hodgson and Bourne, 1988). It is thus of benefit to investigate the highest temperature threshold for larval rearing for the species studies. As a general rule, bivalve larvae reared at temperatures close to their tolerance limits suffer high mortality (Ansell, 1961).
The rearing temperature used for both embryonic development and larvae in Bermuda is similar and was determined through trial and error, rather than a scientific study. The optimal temperature for egg incubation may therefore be worth investigating in these two species, as results in percentage yield of D-larvae varies widely over the years (Table 3.1). However, it has to be noted that bacterial proliferation is associated with high temperatures, and the balance between optimal larval development and low bacterial numbers, needs to be achieved. On the other hand, although scientific investigations may be worthwhile in determining optimal rearing temperatures for larvae, in light of increasing veliger and pediveliger yield, it appears that T= 24 °C yields satisfactory results for both of these scallop species.
3.3.2 Density
Density-dependent mortalities have been described by some workers (Loosanoff and Davis, 1963; Gruffydd and Beaumont, 1972). Initial densities of 5–6 larvae.ml-1 have been described as satisfactory for some bivalve larvae (Jespersen and Olsen, 1982, DiSalvo et al., 1984). Hodgson and Bourne (1988) report that highest survival for P. yessoensis was observed when initial density was 2 larvae.ml-1. Densities used for A. gibbus (15 eggs.ml-1) and E. ziczac (10 eggs.ml-1) fall into the average range used. Velez, Alifa and Perez (1993) maintained the density at 5–10 larvae.ml-1 for E. ziczac throughout its larval life; and do report a low tolerance of this species to high density, especially as they approach settlement. On the other hand, Costello et al.
(1973) incubated A. gibbus eggs at 25 eggs.ml-1 initially, reducing the concentration to 10 larvae.ml-1 at the D-larval stage. It is difficult to evaluate the effect of this initial high density as D-larval yields are not reported.
3.3.3 Salinity
Most rearing of pectinid larvae has been carried out using the local seawater supply, also used for the maintenance of the adults, with salinities within the range of 30–35 ppt. Reduced salinities adversely affect growth of veligers and severely affect embryonic development (Gruffydd and Beaumont, 1972). The degree to which development is affected is species dependent. Embryonic development of the Japanese scallop can take place over the range of about 14-21.5 ppt salinity with a marked reduction in the rate of development at either end of this range (Maru, 1985). Gruffydd (1976) found that survival of Chlamys islanladica veligers over a 24-hour exposure period was little affected by salinities as low as about 21ppt, but markedly reduced by salinities of about 14 ppt with salinities of about 7 ppt causing 100 percent mortalities. Culliney (1974) noted that veligers of the queen scallop, P. magellanicus, could survive for 48 h at salinities as low as 10 ppt, though there was evidence of tissue swelling and the larvae were incapable of normal swimming. Bourne, Hodgson and Whyte (1989) show little effect of salinity on larval growth of the Japanese scallop; and growth rate averaged 6.9 µm per day at a temperature of 18 °C. Pectinid species showing tolerance to differences in salinities, such as the bay scallop, are species naturally found in salinity fluctuating environments. The bay scallop, related to the calico scallop studied in Bermuda, is found in bays, sounds and estuaries, where heavy rains will cause salinity reductions at times to as low as 10–12 ppt (Duggan, 1975). It may explain its steady larval growth rate of 10–15 µm.day-1 when reared between 25 and 30 ppt. On the other hand, A. gibbus is not normally found in the natural environment in low or fluctuating salinity conditions. It can be assumed that its tolerance to lower salinity or to fluctuating salinity will be low, and be reflected in poor larval growth.
Salinity of ambient seawater in Bermuda is constant at 36 ppt throughout the year. There are no adjustments made to the rearing salinity, as both species of scallops used are well adapted to the ambient conditions. Interest was generated in investigating the larval survival and growth of calico scallop larvae with varying salinities for the potential of culturing this species in Gulf of Mexico waters, where salinities can fluctuate daily from 20 to 35 ppt (Norman Blake, pers. comm.). The bay scallop, A. irradians, appears to tolerate these fluctuations in salinity; the question was the degree of tolerance of A. gibbus. Fluctuations could not be simulated at the hatchery in Bermuda; however, three salinities were tested for rearing of larvae, using 3-litre beakers. It was found that, although A. gibbus shows some tolerance to salinity reduced by 8 ppt and even 16 ppt, decreased salinity does seem to a have a negative marked effect in both survival rate and growth. The difference in survival to the pediveliger stage for larvae reared at ambient salinity (36 ppt) and at 20 ppt approximates 12 percent. Both shell and tissue growth of calico scallop larvae were also seen to be negatively affected by reduced salinity, especially towards the end of the larval life. This would most probably affect settlement of these larvae and the post-larval yield and growth of surviving scallops.
3.3.4 Food ration
Molluscan veliger larvae feed by means of ciliary currents on the velum. Hence, once the straight hinge larval stage is reached, the larvae are planktotrophic and feed on unicellular algae. Rates of clearance of particles from suspension are dependent on particle size, concentration of particles, larval size, density of larvae and temperature (Bayne, 1983). Hence, adequate diet in the hatchery environment needs to be assessed in terms of algal species used (see Chapter 2) and amount of algal cells provided (food ration). The optimal or critical cell concentration provided can be defined as that density where all food cells are taken in and no pseudofaeces are produced (Schulte, 1975). Cary, Leighton and Phleger (1981) showed on video films of Hinnites multirugosus larvae (purple-hinge rock scallop) that at high concentrations, mechanical interference was observed, coupled with heavy pseudofaeces production and severe packing of the gut. These authors contend that a finite larval-algal cell encounter/ingestion ratio exists beyond which increasing cell concentrations promote less growth due to the factors previously described. Furthermore, bacterial contamination at high concentrations and the build-up of ectometabolites may render an acceptable diet toxic to developing bivalves (Loosanoff and Davis, 1963).
The protocol used at the hatchery is derived from several in-house trials, as there was little reference in the literature on optimal food ration for calico and sand scallops. Velez, Alifa and Perez (1993) report rearing E. ziczac larvae to the pediveliger stage with a food ration of 30 000 cells.ml-1.day-1 to 70 000 cells.ml-1.day-1 for a larval density of 5–10 larvae.ml-1. With this regime and a constant temperature of 26 °C, the pediveliger stage was achieved in 10–12 days after fertilization; survival rate is not provided. Rojas, Velez and Azuaje (1988) recommend an initial density of 5 larvae.ml-1
for E. ziczac fed a ration of 10 cells.ml-1 with a diet based on Isochrysis aff. galbana (clone: T-Iso) and T. pseudonana (clone: 3H), and end with a density reduced to 2-3 larvae.ml-1 with a ration of 70 cells.ml-1. Preliminary studies in Bermuda (Hohn,
Sarkis and Helm, 2001) on the sand scallop showed that food rations comparable to those of Velez, Alifa and Perez (1993) throughout larval life yielded minimal survival to the pediveliger stage (51 percent of Day-2 larvae), compared to those fed the standard and lower food ration (77 percent of Day-2 larvae). On the other hand, there was no significant difference in shell growth for larvae fed the highest food ration. Costello et al. (1973) reported a daily ration of 60 000 cells.ml-1 for calico scallop larvae throughout their larval life when reared at 10 larvae.ml-1 and a temperature of 23±2 °C; although growth and development to the pediveliger stage was achieved, survival rate is not given. In a hatchery where maximal production is aimed for, it is worthwhile to investigate more closely the larval requirements with size.
3.3.4.1 Effect of food ration on calico scallop larvae
Preliminary studies in 2-litre culture beakers showed that the standard ration initially used at the hatchery was inadequate at certain times throughout the 13 day larval life of calico scallops, in terms of shell growth (Hohn, Sarkis and Helm, 2001). Based on this preliminary study, where three food rations were tested, a second ration schedule was tested using large scale larval cultures (1 000 l), throughout a complete larval cycle during a hatchery run. This second ration schedule provides double the standard amount of algal cells on a daily basis. Hence, control rations ranged from 10 cells.µl-1 to a maximum of 21 cells.µl-1 and tested ration ranged from 20 cells.µl-1 to a maximum of 42 cells.µl-1. Both rations proved adequate in providing nutritional requirements to larvae for growth to the pediveliger stage. The higher food ration seemed to benefit larvae in the middle of the cycle (Day-5 and Day-9), reflected in survival rate only. Nonetheless, a higher yield of pediveligers was obtained from larvae fed the standard (and lower) food ration. Although, differences in food ration were not seen for shell growth, accumulation of reserves reflected in tissue growth was noticeably greater at the end of the larval life in scallops fed the higher food ration. It has been reported that higher reserves in larval life may have an effect on settlement rate, and post-larval growth, influencing the storage of energy reserves and survival of spat (Whyte, 1987). It is therefore advantageous to manipulate food ration to achieve not only high survival rate, but also to ensure enhanced settlement and post-larval growth.
As required food rations are species-specific, consideration must be given to environmental parameters to which the species is acclimated. For example, Bourne, Hodgson and Whyte (1989) reported feeding schedule for P. yessoensis, following numerous feeding trials as being best as follows: Initially, C. calcitrans is fed to veliger larvae and the algal density in the larval tank is 5 000 cells.ml-1. As the larvae grow, additional algal species are fed and the algal density is increased. Such that, by “Day of Setting” (21 days for this species), an algal density of 20 000 cells.ml-1 is maintained in the larval tank, composed of a mixture of C. calcitrans, Isochrysis and T. pseudonana. These authors had the use of a coulter counter, which provided a daily measure of the amount of algae consumed by larvae. In this way, a supplement of algae is added to equate the desired density. Being able to measure consumption of algae also enables monitoring of the health of a larval culture, since healthy larvae will actively swim and graze the algae. Unfortunately, the hatchery in Bermuda did not have access to a Coulter Counter, and it was simply assumed that larvae consumed all of the algae provided on a daily basis. In general, clearance rates increase with the size of the larva at any one particle concentration (Wilson, 1980); however, if particle concentration (or algal density) is above a certain threshold, larvae may reject particles, interfering with feeding and ultimately growth and survival (Bayne, 1983). For this reason, feeding rations are maintained relatively low in Bermuda, to avoid excess uneaten algae leading to increased rejection of algal cells, bacterial proliferation and contamination of larval culture. Food rations provided are within the range investigated by Lu and Blake (1996) for a related species, A. irradians concentricus; these authors determined an optimal concentration of 20 cells.µl-1 for larvae. Finally, care should also be taken to protect larval tanks from bright sunlight, so that algal blooms do not occur and thus create an overfeeding situation.
3.3.5 Culture systems: flow-through vs. static
Traditionally, bivalve larvae are reared in an aerated static system where treated water is changed regularly throughout the larval cycle. Larvae are provided with a daily food supply and are collected prior to every water change. Routine hatchery protocols for the calico and zigzag scallops in Bermuda involve a static system. Although this protocol provides satisfactory results, this method is labour intensive, and requires at times the use of antibiotics to control bacterial contamination within the larval cultures. These two factors play a limiting role in optimizing hatchery rearing of bivalves in general (Southgate and Ito, 1998; Andersen, Burnell and Bergh, 2000). Flow-through systems have been recently attempted with larvae of Pinctada margaritifera L. (Southgate and Beer, 1997; Southgate and Ito, 1998), and with P. maximus scallop larvae (Andersen, Burnell and Bergh, 2000). Design of the flow-through system appears to be one of the major factors in success of larval rearing. It is worthy of investigation as an adequate flow-through system for rearing of scallop larvae would offer a number of advantages over the conventional static culture systems, including reduced physical handling of larvae, reduced labour demand, and a reduced dependence on the use of antibiotics due to an improved water quality. Additionally, in a space-limited environment such as Bermuda, a flow-through system might provide a greater tank capacity to space ratio.
In-house studies in Bermuda investigated various designs for a flow-through system, using the available 200 litre conical tanks. Comparisons in the larval and post-larval yield of calico scallops reared in a flow-through system are made with scallops reared in static systems for the same larval batches. The flow-through system used is illustrated in Figure 3.6. The differences with the more conventional static system lie in the nature of the seawater flow and the procedure for maintenance. In flow-through systems, seawater flows at a steady rate continually, reducing the necessity for frequent and complete water exchange. For this reason, procedures for maintenance of this system revolve around the daily cleaning of the banjos on the outflow, and ensuring a constant water flow. Sarkis, Helm and Hohn (2006) explain in greater detail these differences and the procedure followed in flow-through rearing. This study also provides results of comparative larval and post-larval growth using a flow-through system and a static system.
The flow-through system described here and tested was developed over the course of three years; many preliminary studies were conducted to ensure an optimal water circulation within the tank, a constant water flow, and a steady supply of algal food. The difficulty in designing a successful flow-through system lies in the minimal handling of the system, and lack of assessment of the culture throughout the larval development, rendering difficult the identification of time periods where the culture performs well or poorly. Preliminary studies focusing on the latter, indicated that Day
8/Day-9 after fertilization, when larvae are pre-metamorphic, was a critical period; in that, high growth and survival were obtained in the flow-through system until this point, but a collapse in culture ensued thereafter, related in part to excessive food ration leading to the clogging of the outflow, restriction in water flow, and accumulation of detritus within the tank. For this reason, one water change was performed at this time to allow for fractionation and culling of larvae, in order to optimize survival rates to metamorphosis; and food ration was decreased in the latter part of the larval life; at this time, extreme care was taken in maintaining a constant water flow by repeated cleaning of the outflow banjo. Southgate and Beer (1997) in their trials of flow-through for oyster larvae utilized a larger surface area allowing for outflow of water. Despite
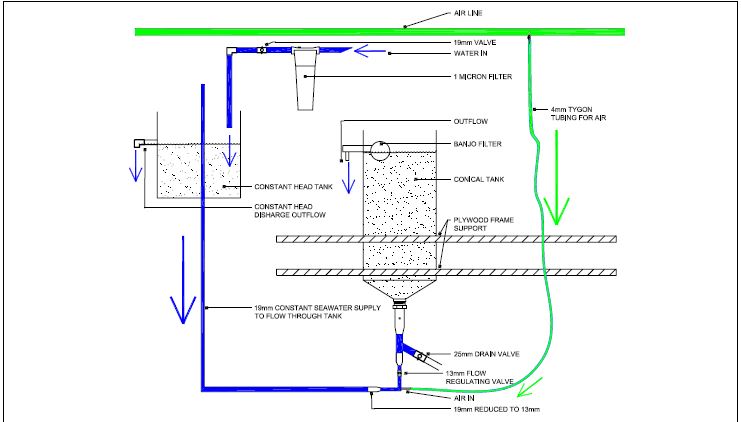
Figure 3.6: Conical tank modified to a flow-through system for larval rearing.
this larger surface area, their results showed a similar trend where larval survival was high during the early part of larval life, and decreased to 7.2 percent survival to the pediveliger stage (Southgate and Ito, 1998). Andersen, Burnell and Bergh (2000) also explained poor larval survival for P. maximus reared in a flow-through system by poor water quality caused by overfeeding. Fractionation and culling of larvae during the water change, is likely to enhance growth and survival of larvae. It has been implied that growth of smaller larvae may be inhibited by larger larvae (Bourne, Hodgson and Whyte, 1989); conversely, the presence of dying larvae may have a negative overall effect on the larval culture, as seen in preliminary experiments towards the end of the larval cycle in the flow-through systems. By incorporating a water change towards the latter part of the larval life, and optimizing the daily maintenance procedure, results of this study demonstrate a comparable performance of the flow-through system in terms of pediveliger yield to that of the static system throughout the larval life regardless of density (Sarkis, Helm and Hohn, 2006).
Pediveliger yields are evidently an important assessment of larval rearing conditions, yet, in an aquaculture operation, the true goal is the yield of fixed spat, ensuring an adequate juvenile production. For this reason, the evaluation of the percentage of spat fixed when reared under different larval conditions was determined in the present study. One of the most critical factors is the accumulation of reserves throughout the larval life, related to food ration in both a qualitative and quantitative sense (Farias, Uriarte and Castilla, 1998). It has been shown that rearing conditions affect storage and utilization of biochemical components, and hence metamorphosis and settlement (Gallager and Mann, 1981). Only one diet was provided in the present experiment for all treatments such that nutritional value was not evaluated; the diet chosen was the standard one utilized at BBSR that has proven adequate over the years for ensuring good spat settlement. Quantitatively, food ration was calculated on the basis of water volume within the tank; such that, in the flow-through system, algal ration was calculated based on water flow, equating to approximately 600 litres of water, as opposed to the 200 litres volume of the tank itself. This food ration provided comparable results when larval density was initially at 8 larvae.ml-1 or 1.6 million larvae in a 200 litre tank; the number of spat fixed approximated 30 percent of the pediveligers for all treatments, in accordance with results obtained for other pectinid species, as for example a 40 percent yield to 1 mm size for P. magellanicus (Couturier, Dabinett and Lanteigne, 1996). On the other hand, it appears that food ration may not have been sufficient, once larval density was increased, for the accumulation of reserves necessary for settlement. The spat yield for flow-through reared pediveligers at a higher density, was much lower than that for those reared in the comparable static system and the standard hatchery system. This density effect was also reflected in the lower shell growth of the fixed spat, as seen when compared to the static system and when compared to the flow-through reared larvae at lower density (Sarkis, Helm and Hohn, 2006).
In conclusion, the results obtained in flow through larvae culture with calico scallops suggest that in resource-limited regions this concept is worth investigating. The benefits lie in the reduced labour involved, the absence of antibiotic use, and the optimization of space availability. These three factors are of economical importance, possibly rendering a hatchery operation more cost efficient.