4.4. TECHNIQUES – SETTING SYSTEMS AND PROTOCOL
The protocol chosen for setting depends partly on space available in the nursery, the period required to remain in the nursery prior to transfer to the field and of the species itself. Furthermore, setting is influenced by several factors such as, the type of setting system and setting density.
Features in setting systems, such as cultch, bacterial films and water flow are believed to stimulate metamorphosis. Cultch is an artificial substrate which increases the amount of surface area in a setting system; if no material is provided larvae can only settle on sides and bottom of tanks, as scallop pediveligers form a byssus attachment to surfaces. It has been found for some bivalve species that if a suitable substrate is not located, larvae can delay metamorphosis and may die (Bayne, 1965). Scallop larvae are believed to be selective about the type of substrate to which they attach before beginning metamorphosis. Several types of material have been tested as cultch for scallop larvae; monofilament, scallop shells polypropylene line, Kinran and Vexar are a few examples. Bourne, Hodgson and Whyte (1989) found Kinran, an artificial fibre made in Japan, to provide optimal results. In addition, the presence of a biofilm on cultch seems to increase settlement, as demonstrated by Parsons, Dadswell and Roff (1993) on Placopecten magellanicus. For this reason, it became standard practice at the Bermuda nursery to soak cultch in filtered flowing seawater for a minimum of 5 days prior to its use for setting.
Other hatcheries use “Chinese hat” collectors for scallop settlement, which are commonly used in the oyster industry (Neima and Kenchington, 1997). These collectors consist of 30 cm plastic disks stacked 6–8 cm apart on a central pipe. A total of 15 collectors can be placed in 1 400-litre tanks.
Other hatcheries have devised horizontal panels with laminar flow for the setting of P. magellanicus larvae (Dabinett, Caines and Crocker, 1999).
4.4.1 Calico and zigzag scallop settlement
Little work has been conducted on the chemical or physical induction of metamorphosis for Argopecten gibbus and E. ziczac. In Bermuda, spontaneous metamorphosis is allowed to take its course and setting is initiated by providing a suitable substrate.
Mature larvae are identified as per criteria outlined in Chapter 3 (see Section 3.4). Set is usually initiated on Day-12 or Day-13 after fertilization for both calico and zigzag scallop species. Mature larvae are collected on sieves ranging from 150 µm to 80 µm as conducted throughout larval rearing (see Protocol–11). If any are collected on 80 µm, health of the culture is visually assessed and culture is either discarded (if mortality is high) or maintained for another two days as larval culture. Spat collected on 150 µm and 120 µm sieves are kept separately; they are suspended in 2 litres beakers and counted. At this stage, larvae are very fragile and fix rapidly on the surfaces of the holding beakers; continuous but gentle plunging is required to avoid fixation of larvae on the surfaces and minimize shell damage. A sample of larvae is kept for determination of shell growth. Counts are done microscopically as outlined for eggs and larvae using a Sedgewick-Rafter Cell (see Protocol–4; Chapter 1). Volume required for setting system is calculated as outlined in Protocol–12 and gently siphoned using a 7 mm Tygon tubing fitted with a stopcock valve into graduated cylinder. Mature larvae are placed into setting systems to undergo settlement, metamorphosis and development into juveniles.
Two methods for setting have been tested at the BBSR nursery. The first, referred to as rapid transfer approach, minimizes the time spat are kept in the nursery to allow transfer to the field within one month of setting; this strategy reduces labour required in the nursery and frees up space for subsequent larval batches. This strategy is useful in areas like Bermuda, where physical space is restricted and larval cycle is short (12–14 days); in this way, production is enhanced by conducting frequent spawning inductions, resulting in a quick turnover of larval batches. One important consideration in rapid transfer of spat to the natural environment is that nursery and ambient temperatures coincide, minimizing stress at transfer. The second method of setting is more conventional and used widely, where spat are set in raceways, on individual sieves, and may be reared up to 5 mm in the same system. This approach (Raceway set) facilitates control of food supply and water flow to spat as well as allows for routine monitoring of spat growth and survival. Although it is more labour intensive with respect to nursery work than the first approach, it allows for a more controlled production of older spat and enhances survival of spat following transfer to the natural environment.
A description of each method is given below.
4.4.1.1 Rapid transfer approach
A detailed procedure (Protocol–12) is given below. Circular fiberglass tanks (450 l) are filled with cultch made of black 3 mm polyethylene mesh, acting as substrate for pediveligers. This mesh comes as a roll and is cut off in 30 cm lengths; fifteen of these strips are tied together and soaked for one week in 1 µm filtered flowing seawater prior to use as settling substrate. All surfaces of the setting tank are covered with bundles of cultch and others are suspended so as to fill the centre of the tank (Figure 4.2). A total of 8 strings are suspended on average. This provides a large surface area for settlement of larvae. Temperature is maintained similar to larval rearing temperature (24±1 °C). Setting (or stocking) density of larvae placed in a setting system is critical. If density is too

Figure 4.2: Cultch made of 3 mm black polyethylene mesh filling 450 litres tanks used for set.
high, rate of settlement, metamorphosis and subsequent post-larval survival declines. Studies on P. yessoensis have shown that low density yields better settlement; satisfactory results were obtained with densities of 0.5-2 larva.ml-1 when set in tanks (Bourne, Hodgson and Whyte, 1989). On the other hand, Neima and Kenchington (1997) found satisfactory results when setting 500 000 larvae in a 1 400 litres tank (equivalent to 35 larvae.ml-1). Stocking density in tanks was not determined specifically for the two scallop species in Bermuda and densities did not exceed 4 larvae.ml-1.
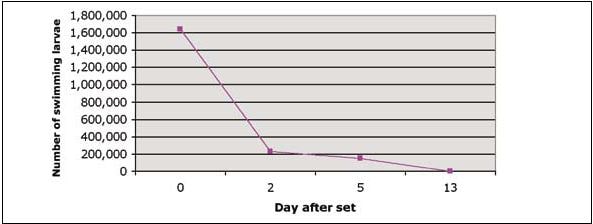
Figure 4.3: Evaluation of calico scallop, A. gibbus, set in 450 litres tanks.
The duration of the setting period, where the maximal number of pediveligers metamorphoses and becomes fixed, is determined by assessing the culture over time. During this time, setting tanks are treated as static larval tanks. Aeration is provided through 7 mm airlines (2 per tank) and algal ration is provided in batches. Larvae are initially fed a mixed algal diet amounting to 18 cells.µl-1 starting day of set; food ration is gradually increased to 24 cells.µl-1 as the number of larvae metamorphose and settle. A detailed table of daily food ration is given in Table 4.1 (see Section 4.5.1).
Although water is exchanged three times a week as for larvae, the procedure differs in that setting larvae are not removed from the setting tank, and as they need to be continually submerged, the water level in the tank is maintained constant. A 20 mm inner diameter flexible hose is connected to a supply of 1 µm filtered heated seawater to provide continuous flow to the setting tank. Water is passed via the outgoing valve at the bottom of the tank and any swimming larvae (or not fixed) are collected on a sieve in a submerged tray as for larvae. The valve is opened to provide a similar outflow to the inflow supplied to the tank; in this way, water level remains constant throughout the water exchange. This water exchange lasts for a period of 1 hour in order to ensure complete water exchange of the whole tank. Larvae collected on the sieve are washed into a beaker, counted using a Sedgewick-Rafter cell and measured with an ocular micrometer using a compound microscope. Larvae collected are for the most part those which had not fixed onto the substrate and are classified as “swimmers”. Collected larvae are re-suspended into the setting tanks, unless a high percentage of dead larvae are seen in the sample. Setting period is considered complete when swimmers are no longer seen in the collected sample.
Towards the end of the setting period (sixth day), temperature acclimation to ambient seawater is achieved by decreasing set temperature by 1 °C every two days. Once maximal number of larvae are set (or 10 days after set for these species), the setting system is changed to an open flow seawater system, where seawater is filtered to 1 µm and supplied continually to the tanks; an air-lift driven recirculation system (illustrated in the technical drawing diagram – 5/Pg19B) enhancing water exchange within the tank, is initiated. Algal food supply is provided continuously over 24 hours by drip
feed. Young spat are reared in this way for a further 20 days. After which, they are ready for transfer to growout sites.
For both scallop species reared in Bermuda, a period of 10 days is selected as standard for duration of the setting period. As seen in Figure 4.3, monitoring of 450 litres sets has shown the absence of “swimmers” in the collected sample by Day-13. It is assumed that larvae are either fixed or dead by this time. Note: shell length of larvae collected during setting did not change (198±10.2 µm) implying that larvae collected are those with slow development.
PROTOCOL–12
SET OF MATURE LARVAE IN 450 LITRES TANKS – RAPID TRANSFER APPROACH
1. Five days prior to anticipated setting day, prepare cultch. Cut 30 cm strips of black 3 mm polyethylene mesh and tie in bundles of 15. Soak in 1 µm filtered flowing seawater to develop a biofilm.
2. Fill 450 litres tanks with bundles of 3 mm polyethylene strips. Cover the bottom of tank, and use re-circulation pipe system to suspend bundles so as to fill centre and top of tank.
3. Fill tank with heated double 1 µm filtered seawater. Temperature in 450 litres tank should be same as larval rearing temperature (24 °C).
4. Prepare aeration as for larvae using two 7 mm Tygon tubing connected to airline and reaching to bottom of tank. Place two lines on opposite side of tank.
5. Setting Day referred to as Day-0 of set.
6. Collect mature larvae on 150, 120 and 80 µm sieves. Only set those collected on 150 and 120 µm. Suspend separate fractions in 2 litres beakers. Keep larvae in suspension by using a gentle up and down motion with plunger. Motion for mature larvae has to be continuous during sampling and prior to setting as they will attach to sides of beaker quickly.
7. Count mature larvae using a Sedgewick-Rafter Cell. Calculate number of larvae so as to distribute in 450 litres tank at density not exceeding 4 larvae.ml-1.
8. For calculation: Do Triplicate counts and take average count (if culture very dense, take 500 µl aliquots instead of 1 ml).
Example:
- Average count: 151 eyed larvae in 500 µl = 302 larvae in 1 ml
- Total volume of pool (in beaker or in cylinder) =3 litres = 3 000 ml
- Total number of larvae in pool: 302 x 3 000 = 906 000 larvae
- Setting in 450 litres tanks at a density of 1.5 larvae per ml:
- Total number of larvae in 400 litres = 400 x 1 500 = 600 000 larvae
- Since 302 larvae in 1 ml, for 600 000 larvae need to siphon:
600 000/302 = 1 986 ml
9. Pass mature larvae over 300 µm sieve. Keep size fractions separate if possible (150 and 120 µm) and set in different tanks.
10. Maintain temperature in tanks to 24 °C by two immersion heaters.
11. Feed set larvae as per larval rearing, as batch feed.
12. Water exchange conducted every two days as for larvae (Monday, Wednesday and Friday).
13. For water exchange: Prepare shallow tray and sieve (dependent on size set – for 120 µm set use 120 µm sieve; for 150 µm set use 150 µm sieve) to collect any swimming larvae. Connect 20 mm flexible hose to outgoing valve of 450 litres tank and place into sieve. This is same procedure as for collecting larvae outlined in Protocol–11.
14. Connect 20 mm ID flexible hose to treated seawater line (double 1 µm filtered seawater) and start gentle flow.
15. Partially open collecting valve of 450 litres tank and ensure flow of water is into sieve.
16. Ensure to maintain larvae and substrate in water by adjusting incoming flow to be similar to outgoing flow.
17. Exchange water for 40 minutes.
18. At the end of exchange, close collecting valve and stop incoming flow.
19. Count number of larvae obtained in sieve using Sedgewick Rafter Cell; record number of dead and swimmers.
20. If live larvae outnumber dead larvae in collected sample, re-suspend in tank, passing over 500 µm sieve to eliminate any debris. If not, discard.
21. Feed daily as per Table 4.1. Food ration calculated as:
Ration (ml) = (cells.µl-1 to supply/total algal count in cells.µl-1) x water volume (l) x 1 000 22. On Day-6, begin decreasing temperature of seawater so as to reach ambient seawater temperature by Day-10. Decrease by 1 °C every two days.
23. Continue rearing in this system until Day-10.
24. When maximal number of larvae is considered fixed (Day-10), begin re-circulating system and change static system to flow-through system. Connect hose to incoming ambient 1 µm filtered seawater supply and open drain valve.
25. Algal feeding supply now provided through 20-litre carboy placed on drip-feed. Carboy cleaned and filled daily.
26. Maintain in open and re-circulating system until transfer to field.
4.4.1.2 Setting density for raceway system
A second approach to setting makes use of raceway systems. Mature larvae are set on sieves, meshed with 120 and 150 µm Nitex material; sieves are suspended in raceway troughs as shown in the technical drawing diagram – 3/Pg15B. Density of larvae/spat per sieve is critical at all stages of this procedure; it is monitored weekly in terms of biomass (grams wet weight.sieve-1) and re-adjusted if needed. In order to optimize percent settlement, initial stocking density for mature larvae set in sieves was investigated for E. ziczac at BBSR nursery. Three densities were tested: 25 000, 50 000 and 100 000 pediveligers per sieve. As expected, decreasing initial density results in an increased percentage of fixed spat; it is evident that a balance needs to be achieved at the nursery between an optimal spat yield and usage of space. As a stocking density of 25 000 is not a practical solution in a space-limited hatchery like the BBSR modular hatchery, standard stocking density in Bermuda averages 50 000 larvae per 25 cm diameter sieve. Stocking density never exceeds 60 000 larvae per 25 cm sieve or 30 larvae.cm-2.
4.4.1.3 Raceway set
The standard protocol for setting spat on sieves in raceways is given in Protocol–13. The calculated number of pediveligers is quickly transferred into prepared 150 µm or 120 µm sieve following counting (Figure 4.4). Once again mature larvae are allowed to set with minimal flow and no other disruption for ten days. Food supply is provided from 20-litre carboys, placed on 24 hours drip-feed; this accounts for 50 percent of the ration. The second half of the ration is distributed in the sump tank and distributed through the re-circulating system. Sieves are suspended in the raceway, set on a semi
recirculating system (described in Section 4.1), with inflow of 1 µm filtered seawater at a rate of 3 l.min-1. Semi-recirculation ensures that all algae supplied is consumed, and allows for some temperature control of seawater. Similarly to the procedure for 450 litres tank set, mature larvae are set at a temperature of 24 °C as during larval rearing.
Temperature is maintained by two immersion heaters placed in the sump tank used for recirculation.
Monitoring of several larval batches has shown that mature larvae set within 10 days of being provided with a substrate, as seen in section on 450 litres tanks above. Following this time period, any larvae collected are dead or poorly developed.

Figure 4.4: Mature larvae set on meshed sieve suspended as downwelling system in raceway.
Following this setting period flow and food ration are increased and the protocol for post-larval rearing in terms of cleaning, monitoring and thinning sieves for optimal post-larval yields is initiated. Food ration throughout this stage in the indoor raceway system is provided in Table 4.1. After Day-10, flow of raceways is set according to biomass in the system; as a rule flow is set between 25 ml.min-1.g-1 of spat (wet weight) to 50 ml.min-1.g-1 of spat. Note: Flow can be adjusted depending on the amount of live algae available; if fewer algae are available, decrease the flow so as to decrease volume of water. Keeping a record of biomass and maintaining adequate biomass per sieve and in the system becomes critical towards the third week (Day-22) following set when spat begin a rapid increase in growth. If this biomass per sieve, and per system, is not controlled, high mortalities will ensue coupled with slow shell growth.
Spat are maintained in this system up to 2 months, or until they reach 3.5 mm in shell height. At which time, they are ready for the secondary nursery stage – i.e. transfer to the outdoor raceway for further growth to a minimum of 5 mm in shell height. Adherence to a strict protocol (see later Section 4.5.3) facilitates maintenance of the entire system on a weekly basis, ensuring adequate biomass for growth. Collection of spat for the next stage is simply done by washing them off the sieve with a gentle jet of seawater.
PROTOCOL–13
SETTING MATURE LARVAE IN RACEWAY – MAINTENANCE AND CARE
Setting Day = Day-0 of set.
1. Clean 30 cm sieves by wiping off sides and mesh with cloth and chlorinated water. Rinse thoroughly with fresh water using a strong jet on mesh to clear all openings. 2. Clean raceway thoroughly following directions in Appendix 19.
3. Fill raceway system (including sump tank) with 1 µm filtered seawater. 4. Once filled, turn off incoming flow and place raceway on re-circulating system only. 5. Place two immersion heaters in sump tank to increase temperature to 24 °C. Monitor temperature.
6. Pool larvae and pass through 150 and 120 µm sieves. Re-suspend each fraction in a graduated container and count as per Protocol–12.
Chapter 4 – Nursery: facilities and culture of post-larvae 107
7. Calculate volume required as per Protocol–12.
Example:
Average count: 302 larvae per ml
For Setting in 25 cm diameter sieve at a density of 50 000 larvae per sieve: 50 000/302 = 165 ml
8. Distribute larvae into 150 or 120 µm raceway sieve dependent on size at a density not exceeding 50 000 larvae per sieve.
9. Partially open incoming seawater flow so as to obtain a flow of 3 l.min-1. This should provide a total volume of 1 200 litres for entire raceway system.
10. Restrict flow (drip-like) to individual sieves on setting day so as to leave larvae to settle without any surface agitation.
11. Calculate food ration (Protocol-10 and 12). Distribute 50 percent in sump tank. Distribute remainder in 20-litre of carboy and top up, with 1 µm filtered seawater. Adjust flow of carboy to raceway pipe to create 24 hours drip-feed. Use Table 4.1 for food ration.
12. Fill 1 carboy per raceway. If two carboys are used, total volume of algae required is divided into two.
13. Record number of larvae set in sieve in raceway check sheet (see Appendix 18) and label sieve with date and species.
14. Conduct daily check of flow, temperature and algal ration provided on raceway check sheet.
15. On Day-2 – Increase flow slightly too fast drip.
16. On Day-6 – Start decreasing temperature to ambient seawater temperature (1 °C every two days).
17. On Day-8 – Clean raceways. Transfer sieves to saltwater table filled with heated 1 µm filtered seawater. Clean raceway as detailed in Appendix 19. DO NOT CLEAN SIEVES; DO NOT DISTURB SETTLING LARVAE.
18. On Day-10 – Assess number of swimmers: Prepare a tray (clean with chlorinated water and rinse); fill to 1/4 with filtered seawater. Remove sieve from raceway and gently wash inside mesh and sides into beaker. DO NOT BLAST POST-LARVAE OFF SCREEN; this will damage and cause breakage of shell and mortality. Place sieve in tray so that fixed spat remain submerged. Count swimmers and/or dead larvae using 1 ml aliquots with Sedgewick Rafter Cell. If many swimmers, re-suspend in sieve. Put sieve in raceway and adjust flow to maximum. Collected sample should contain few swimmers and mainly dead larvae. It is difficult to estimate the number of spat set at this time due to small size.
19. From Day-10 on begin routine cleaning of raceway system once a week (see Appendix 19).
20. By Day-22, some crowding may be observed, and spat are seen climbing up the sides of the sieve. Thinning of sieves should be initiated (Table 10).