4.5 TECHNIQUES – POST-LARVAL REARING REQUIREMENTS
4.5.1 Food ration for spat
Observations suggest scallop spat may use an alternate type of feeding between the time the velum is lost and the gill filaments are able to take over the feeding process. During this time, spat may not be able to filter feed and instead may feed on particulate material adhering to surfaces. The foot may be used for this feeding process.
For this reason, culturists differ in their feeding protocols at time of setting. For P. yessoensis, Bourne, Hodgson and Whyte (1989) fed setting larvae similarly to other larvae, at a level of 20–25 cells.µl-1 with a mixed diet of algal species (Isochrysis sp., Chaetoceros calcitrans and Thalassiosira pseudonana); these authors report only a slight removal of algal cells from the water column, implying little algal consumption.
Investigating optimal food ration for fixed larvae has been attempted several times at the hatchery in Bermuda. It has proven difficult to assess optimal food ration with newly settled spat. Difficulties lie in the assessment of spat without causing shell damage or mortality, and in setting-up an experimental system, which mirrors a larger nursery system. The method for assessing the effect of food ration in spat was developed over the course of several studies; it was found that due care must be taken for the even distribution of food supply throughout the experimental system. Preliminary studies seem to imply that increasing food ration has little effect on shell growth in the first 14 days of post-larval life. A 14-day experiment, using beakers, was conducted with newly settled spat (Day-10 after set). Some difference was seen, namely in survival rate of post-larvae, with food ration; where, high food ration is associated with low survival rate. On the other hand, no trend was seen between shell growth and food ration. It appears that food ration is not critical to growth of settling post-larvae, however, several subsequent studies indicated that older post-larvae of 1.7–3.0 mm in shell height (approximately 1 and 2 months old spat, respectively) do benefit from an increased food ration. They demonstrated that spat >1.5 mm in shell height benefit from an increased daily food ration, reflected in increased shell and tissue growth, and an associated high survival.
The point where food ration supplies exceeds the filtering and ingestion capacity of scallop spat was not reached in these experiments. Nonetheless they provided the basis for the food ration protocol used at the BBSR nursery.
4.5.1.1 Standard food ration protocol for calico and zigzag scallops
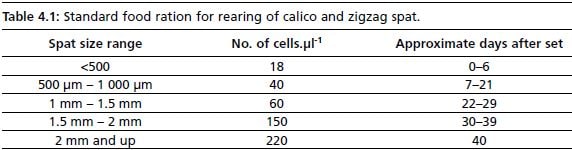
Standard food ration given for calico and zigzag scallops follows the schedule given below. As seen above, food ration does not need to be high in the first 7 days of growth; it is steadily increased as spat grow. An approximative seeding schedule is given in Table 4.1; this is dependent on batches and may vary slightly with different larval cycles.
Table 4.1: Standard food ration for rearing of calico and zigzag spat.
4.5.2 Strategy for efficient use of space in rearing spat
The main protocol in rearing post-larvae makes use of the raceway system, as opposed to the 450 litres tanks. The reason for this is related to ease of handling of spat as they grew; it proves more labour intensive to remove spat from cultch than to rinse them down from the sieves. Although this is true for both E. ziczac and A. gibbus, it may not be for other species, which may detach more easily. Furthermore, there are studies showing the successful use of chemicals for detaching of spat from cultch. The setting of mature larvae in 450 litres tanks is therefore done in Bermuda when there is an overflow of pediveligers, which the raceway system cannot accommodate. It is however, anticipated that the nursery in Bermuda will remove the 450 litres tanks and replace them with a second indoor raceway system.
Due to the small size of the hatchery in Bermuda and hence of limited larval production at any one time, the goal of the nursery area is to ensure optimal juvenile production by enhancing survival rate during the following three stages: 1) From settlement to 3 mm shell height; 2) Transfer to the field; and 3) In the first month of growout in the natural environment. In this way, efficiency is maximized despite the small production capacity of the facility. Survival and growth following settlement to 3 mm size seem to be very dependent on initial stocking density, food ration, available surface area and minimal handling. On the other hand, for older spat, biomass per sieve and associated water flow, appears most important. Finally, in order to minimize mortality during and following transfer to the natural environment, it has been observed that increased size of spat at transfer enhances survival. For these reasons, an additional raceway was built outdoors (see Section 4.1), maximizing available space for growing of spat to a larger size prior to transfer to the field. When spat reach 5–7 mm in shell height, they can be directly transferred to 3 mm pearl nets, and hung on longlines. Survival rate after one month of transfer averages 90 percent. The additional exterior raceway system has for its main purpose the rearing of spat from 2 to 5 mm.
4.5.2.1 Characteristics of outdoor raceway
The outdoor raceway system differs to that of the indoor raceway system in the following: Sieve characteristics, seawater treatment and algal food composition. Sieves used are of a greater surface area (696 cm2) than those used indoor to accommodate a larger biomass of spat. They are meshed with green collector bag mesh (1.2 mm on diagonal), ensuring greater water flow. Sieves are set on an upwelling system, reducing clogging of mesh with detritus.
Seawater supply comes directly from the pump house (see technical drawing – page 16A) and is only filtered through the sand filter; this allows supply of additional nutrients to the spat, such as naturally occurring algal species.
Algal food supply is provided from dry algae cultures, purchased at Reed Mariculture Inc. Three species of algae are provided daily by weight of algae. Appendix 21 gives the procedure for preparation of algal food ration for outdoor raceway. The dry algal mixture is diluted in a small volume of seawater and transferred to a large 150 litres reservoir filled with seawater and connected to the outdoor raceway. Algal supply is administered via the seawater to each raceway channel; algal mixture is supplied to each sieve via an upwelling system. This system most probably does not provide uniform supply of algae to each sieve, dependent on the position of the sieve in the raceway. For this reason, sieves are rotated weekly to ensure uniform supply of food (Protocol–15).
There is less control in this outdoor raceway system than in the indoor raceway, in terms of food composition and seawater treatment. This does not seem a deterrent to successful rearing, as with age spat become more tolerant to varying environmental conditions.
The role of the outdoor raceway is to maximize growth enabling the transfer of spat directly into 3 mm pearl nets and enhance subsequent survival in the natural environment. For this reason, several studies were conducted to investigate the optimal size for transfer of spat to the outdoor raceway, and procedures for optimal shell growth in this system. Zigzag scallop spat of two size fractions (<3.5 mm and >3.5 mm) were reared in the two different raceway systems for a period of 4 weeks. Preliminary results indicate that the smaller size fraction fared best in the indoor raceway system; this system is more controlled in terms of water filtration, distribution of algal ration, temperature and quality of algae provided. On the other hand, as spat grow, they become more tolerant to varying environmental conditions; this was seen in the shell growth rate of older spat reared in the outdoor system. In fact, the indoor system appeared limiting to the growth of larger spat, and these showed a faster growth rate in the outdoor system. This may be related to nutritional requirements and the lack of fine filtration in the outdoor system, which may provide a nutritional supplement to the spat. On the other hand, the outdoor system did not support good survival of spat >10 mm; this is most probably also due to increasing nutritional requirements exceeding the supply provided in this system.
The efficiency of the raceway system in rearing spat tolerant to subsequent transfer to the natural environment for growout was also seen during this study. No subsequent mortality was recorded following transfer to the field in 3mm Japanese pearl nets.
This preliminary experiment was designed to give an indication of growth and survival of spat in the raceway systems; although it is not comprehensive, it does help in defining a strategy for the overall nursery system and allocate space logically. From thereon, it was concluded that spat larger than 3.5 mm would be either moved to outdoor raceway for rearing to 10 mm prior to transfer to the field, or transferred to the field in a tray system, depending on hatchery capacity at the time.
4.5.2.2 Density effect on spat growth
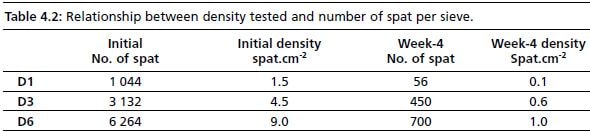
Rearing scallops differs from the rearing of other bivalves, namely oysters and mussels, in the required surface area for optimal growth. Scallops do not cluster as some other species do. This explains observations of scallop post-larvae seen “climbing” the sides of the sieve as they grow, distributing themselves in such a way that they are not on top of one another. As space appears to be a factor for growth, effect of density on shell growth of older spat (>3.5 mm) was investigated. Density was expressed as grams wet weight.sieve-1, as this is a more practical assessment than counting of spat. The latter can be useful at times, however, and is discussed later. Densities tested were selected on the basis of percent coverage of sieve; three densities were tested 7.2, 21.6 and 43.2 grams wet weight.sieve-1. Although survival was not affected within the density range tested, scallop spat reared at lower densities clearly benefit in shell and tissue growth. Practically, low density is difficult to sustain in a compact environment, as is the modular hatchery. As can be seen from Table 4.2, the lowest density translates into 56 spat per sieve at the end of week 4. As it is not cost efficient to culture spat at this density, a balance between space availability and optimal growth needs to be achieved.
Table 4.2: Relationship between density tested and number of spat per sieve.
4.5.3 Raceway weekly maintenance
The studies conducted on post-larvae and older spat, although not comprehensive, do provide baseline data for management of the nursery area. From these stemmed a protocol, strictly adhered to, in order to maintain correct biomass within raceway systems, thus enhancing survival in the early spat stages, growth rate, and subsequent survival following transfer in the field. Monitoring is done in terms of biomass rather than number of spat, as obtaining wet weight is less time consuming than counting spat per sieve. Nonetheless, at times it is useful to translate production into number of spat, and Protocol–14 gives the procedure used for counting spat directly on a sieve. A grid was made at the BBSR nursery and laminated to be somewhat waterproof; this grid is placed under a sieve of spat for counting (see Appendix 20). Within the nursery routine, a schedule for cleaning of raceways is incorporated, as cleanliness for post larvae is as critical as when rearing larvae.
Raceways, associated tanks and sieves are cleaned once a week following the protocol outlined in Appendix 19. The first cleaning following set is done only on the raceway itself (see Protocol–13). Sieves are transferred into a holding area filled with 1 µm filtered seawater and left undisturbed during the period of cleaning. On the second week of set, a regular schedule of cleaning raceways and individual sieves is initiated; sieves are cleaned with a gentle jet of seawater, care is taken to not damage the shells, but detritus is removed to allow for better flow through the mesh. Cleaning of raceways is conducted on Tuesdays allowing for routine hatchery operations on Monday, Wednesday and Friday. Daily monitoring of raceway system and post-larval culture requirements are checked and recorded on a raceway check sheet, a sample of which is given in Appendix 18.
As post-larvae grow, total biomass in the raceway system increases; adjustments in algal food ration, water flow and available surface area are required. Food ration is discussed above and given in Table 4.1 for the indoor raceway. Total seawater volume for the indoor raceway, when used as a semi-recirculated system, is maintained at 2 320 l per day, irregardless of biomass; this is calculated by incorporating incoming flow of 3 l.min-1, volume of reservoir (220 l) and overflow discharge of 1.25 l.min-1. At this stage, algal food ration is based on volume of water, rather than on biomass. On the other hand, seawater flow for the outdoor raceway, set as an open system, is based on spat biomass in the system (50 ml.min-1.g wet weight-1, equating 2 l min-1 for each raceway channel). Care is taken to minimize flow so as to avoid too rapid a discharge of algae. Food ration is provided as commercially purchased dry algae, diluted with seawater in a 150 litres tank and pumped to each raceway channel. Protocol–14 provides details on procedures followed for rearing of spat in the outdoor raceway.
Weekly cleaning of raceway is combined with biomass check starting Day-22 after set. This protocol also allows for monitoring of growth, and size fractionation of spat in the latter stages of the nursery.
PROTOCOL–14
REARING SPAT IN OUTDOOR RACEWAY
1. Clean raceway once a week (generally on Tuesday)(see Appendix 19).
2. Clean one channel at a time; transferring sieves to other channel during cleaning. 3. Use chlorox and a scrub brush for removing all detritus. Use a bottle brush for cleaning pipes, inserting it through cleaner valves.
4. Clean algal system by chlorinating 150 litres reservoir and pumping chlorinated water through algal supply lines. Be careful not to pump chlorine into raceway channel with spat.
5. Wash sieves gently with saltwater so as to remove silt. Do this twice a week (Tuesday and Friday). System clogs up more quickly as water is not fine filtered.
6. Rotate sieves once a week (Tuesday) so that first three sieves are moved to last three positions to ensure equal supply of food for all.
7. Maintain 25 grams biomass per sieve. Check on weekly basis (Wednesday)(see Protocol–15).
8. Daily, ensure that algal tank does not drain completely and that pump does not run dry. Check algal level before leaving in the evening. It should be 1/3 down. If not, increase algal supply to raceway.
9. For feeding: Turn pump off. Drain algal tank by opening union at bottom of tank (see technical drawing – 1/Pg17) and clean algal tank thoroughly with chlorox and fresh water. Rinse tank with ambient seawater. Close union and fill tank with ambient seawater. Turn pump back on once tank is filled to 1/3.
10. Remove prepared aliquots of dry algae (use a mixture of 3 species) and dilute in 1 litre of seawater. Let sit, and add to 150 litres tank. See Appendix 21 for preparation of dry algae.
11. Adjust supply to raceway using stopcock valves. A fast drip is usually adequate to empty 150 litres tank in 24 hours.
4.5.3.1 Maintaining a critical biomass
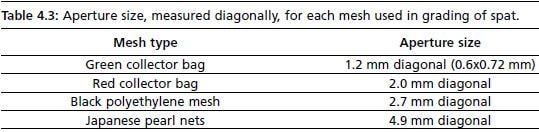
The terms “thinning” and “grading” are used throughout the following section. “Thinning” refers to assessment of biomass (total wet weight) per sieve, and redistribution of spat. “Grading” refers to fractionating sizes of spat using various mesh size.. There are mechanical graders available commercially, and are useful for large operations. In smaller hatcheries, graders can be made using a series of 30 cm diameter sieves, meshed with polyethylene material of various apertures. In Bermuda, collector bags used for natural spat collection in the field, and Japanese pearl nets used for growout were cut and used as mesh for graders. Table 4.3 shows the aperture of each mesh size used; aperture size was measured diagonally as it is the largest opening through which spat may pass.
Table 4.3: Aperture size, measured diagonally, for each mesh used in grading of spat. Mesh type Aperture size
A general schedule for thinning and grading of early spat, reared in the indoor raceway system, is given in Table 4.4, and the procedure itself is outlined in Protocol–15. Thinning of spat, or first control of biomass, is usually required by Day-22 after set. Spat are washed off the sieve using a gentle jet of filtered seawater and collected into a tarred mesh square of appropriate size. At Day-22, it may be difficult to detach all spat from sieve; it is best to leave those that stick on, rather than damage by detaching. Collected spat, enclosed in the mesh, are blotted dry and weighed on a Sartorius balance (±0.01 grams) (Figure 4.5). Spat from same set (and size) are divided into
Table 4.4: Procedure for maintenance of biomass in raceway system and transfer to outdoor raceway. Sieve size for indoor (532 cm2) with 150 or 120 µm mesh. Sieve size for outdoor (696 cm2) with 1.2 mm (green) mesh.
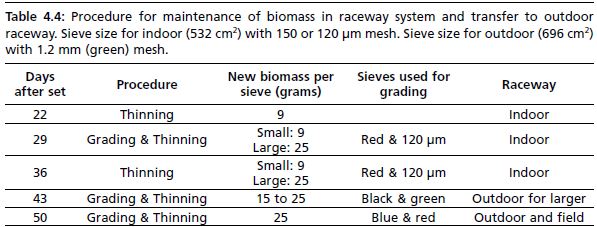
9 grams wet weight aliquots and re-distributed into sieves. A 9-gram biomass at Day-22 is equivalent to 60 percent coverage of the sieve; it was found that this provided adequate surface area for growth of spat, and this percent coverage became the standard objective for re-distribution at every grading/thinning session. At Day-22, size fractionation is not found necessary. On the other hand, by Day-29, size range of spat increases, and pooled spat are passed through a red mesh sieve and 120 µm sieve. Those collected on the larger red sieve are ready for transfer to the outdoor raceway. On average, this amounts to approximately half of spat. Spat are weighed and redistributed into sieves; biomass per sieve does not exceed 9 grams for the smaller size fraction (indoor raceway), and 25 grams for the larger size fraction (outdoor raceway). The next thinning and grading session is usually two weeks later (Day-43), where all spat become large enough to be transferred to the outdoor raceway at a density of 15–25 grams per sieve. On Day-50, or <2 months after set, spat are graded using black and red sieves; those spat collected on black mesh are ready for transfer to pearl nets. Others are re-distributed into sieves at a density of 25 grams per sieve.

Figure 4.5: Weighing spat on a Sartorius balance (±0.01 gram).
PROTOCOL–15
WEIGHING AND COUNTING OF SPAT FOR THINNING AND GRADING
1. Clean raceway once a week (Tuesday)(see Appendix 19).
2. Thin and grade sieves once a week (Wednesday) starting Day-22.
3. Preparation: Clean 20 mm hose and connect to ambient 1 µm filtered seawater. 4. Clean saltwater table with chlorinated sponge, rinse thoroughly with fresh water and 1 µm filtered seawater.
5. Let filtered seawater flow in table.
6. Place measuring grid (Appendix 20) on saltwater table and place sieve on top. 7. Identify 10 squares (each 1 cm2) for counting. Ensure that selected squares represent all areas of the sieve (corners, centre, borders).
8. Count number of spat per cm2. Average number of spat per cm2. Estimate total number of spat as follows:
Total number of spat in sieve = Average (spat per cm2) x SA of sieve
(Note: SA for 25 cm sieve = 532 cm2; SA for 30 cm sieve = 696 cm2)
9. To obtain biomass: Prepare several pieces of 15 cm2 mesh (aperture size depends on age of spat – see Table 4.4).
10. Tare one piece using a balance (±0.1 gram).
11. Collect sieve from raceway, and using gentle jet of seawater, gather spat to corner of sieve.
12. Pass spat through two sieves for grading (see Table 4.3). Repeat with remaining sieves. Keep two size fractions separate and hold graded spat in two extra sieves sitting in tray with 5 cm of filtered seawater in bottom.
13. When all spat are graded, transfer aliquot of spat into tarred mesh piece using a spatula or small spoon. Wrap spat in mesh and blot dry on a piece of paper towel.
14. Weigh spat on same balance. Record.
15. Clean sieve thoroughly with chlorinated sponge for sides and strong jet of seawater through mesh to remove all debris. Suspend in raceway.
16. Weigh out aliquot of spat required for adequate coverage, dependent on age (see Table 4.4).
17. Transfer spat in cleaned sieve.
18. If require measurement of spat, use ocular micrometer on compound microscope at magnification of 4 until 2.5 mm in shell height. After which, use Vernier callipers (±0.1 mm).
19. Calculate total biomass in raceway and adjust flow based on 50 ml.min-1.g-1.
20. Feed according to Table 4.1 and Protocol–13 for indoor raceway and Appendix 21 for outdoor raceway.
4.5.4 Shell growth of calico and zigzag scallop spat
Post-larval shell growth is similar for calico and zigzag scallops. Figures 4.6 and 4.7 show shell height for both species when reared according to the protocols given above. In short, within 2 months of set, >90 percent of spat can be transferred to the field in 3 mm pearl nets. For additional assurance of post-transfer survival, spat can be maintained a further two weeks in the outdoor raceway; thus transfer of spat to pearl nets can be done 2.5 months after set with spat averaging 9 mm in shell height. The total time in hatchery and nursery prior to transfer to the natural environment does not therefore exceed 3 months. As for survival rate, mortality incurred following metamorphosis, setting of larvae and early post-larval growth, results in an average of 20 percent survival of larvae set to time of transfer (Figure 4.8). Growth rate given is associated with temperatures shown in Table 4.5.
In order to estimate total production of hatchery in terms of numbers, it is best to establish a baseline relating wet weight of spat to shell height and age. In this way, for subsequent batches, production can be estimated by extrapolating known biomass. This was done at the BBSR nursery for the calico scallop and is shown in Figure 4.9. In this way, if total biomass of Day-50 spat is known and growing at a known rate, the total number of spat produced can be calculated. This is evidently dependent on rearing conditions and growth and should be established for each nursery.
Table 4.5: Ambient temperature recorded in raceway systems in Bermuda during post-larval growth. Range represents monthly change.

Transfer of spat from the nursery to the natural environment for growout is described in Chapter 5.
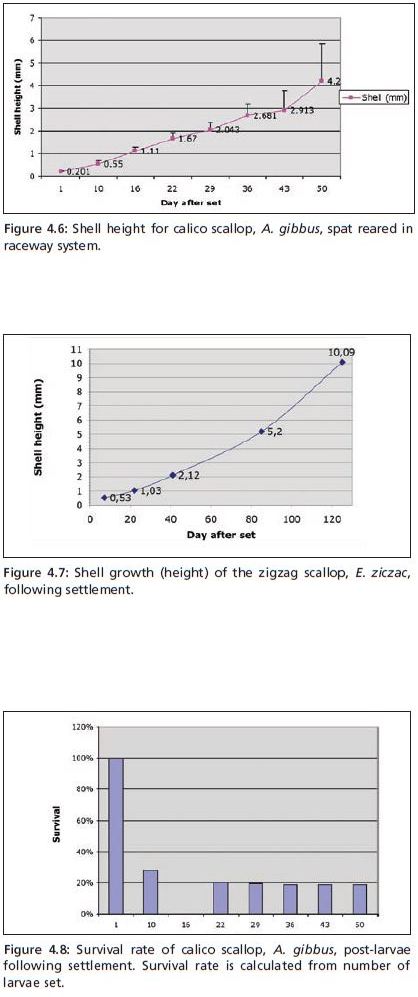
Figure 4.6: Shell height for calico scallop, A. gibbus, spat reared in raceway system.
Figure 4.7: Shell growth (height) of the zigzag scallop, E. ziczac, following settlement.
Figure 4.8: Survival rate of calico scallop, A. gibbus, post-larvae following settlement. Survival rate is calculated from number of larvae set.
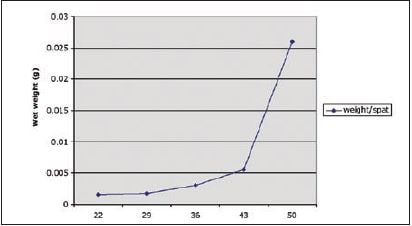
Figure 4.9: Wet weight of calico scallop, A. gibbus, spat (gram per spat) as determined during the nursery stage (n= 100).