3.3.2 Details of intermediate-scale culture operation
The complexity of the culture operation depends on the requirement for algae and the cost constraints within which the system needs to operate. In the simplest form the culture system may be just a scaled-up version of the starter cultures, using 2 l to 25 l flat-bottomed, glass flasks or carboys.
These are part filled with the culture medium – in this case sterile, nutrient-enriched seawater – and then they are inoculated with the required species and aerated with a mixture of 2% CO2 carried in compressed air. The carbon dioxide is from a bottled gas source with gas pressure and flow regulation. This is to provide the carbon source for photosynthesis and to control pH within the range 7.5 to 8.2. The air/CO2 mixture is filtered through a 0.2 ?m porosity cartridge or membrane filter to remove the majority of air-borne contaminants and competing microorganisms. Examples of this type of system are illustrated in Figure 18. The culture medium is prepared from filtered or sterilized seawater.
There are various options for culture water treatment:
a) either the seawater is filtered to remove bacteria using 0.22 or 0.45 ?m membrane cartridge filters, or,
b) it is batch or continuously pasteurized at 65 to 75°C or,
c) it is autoclaved at 1.06 kg per cm2 for 20 minutes (After autoclaving the medium must be allowed to stand for 2 days in a suitable container closed from the atmosphere). Or,
d) it is chemically sterilized with sodium hypochlorite solution at 25 mg per l free chlorine (by adding 0.5 ml of domestic bleach – 5% sodium hypochlorite – per l of filtered seawater). Before use, the residual free-chlorine is neutralized by adding an excess of sodium thiosulphate solution (50.0 mg per l) prepared in distilled water.
Note: Methods (a) and (c) are most commonly used for small-scale culture preparation; (b) and (d), after prior filtration to 1 or 2 µm particle size, for large-scale culture.
After the sterilizing treatment, nutrient additions are made. Details of the nutrient enrichment used at the Ministry of Agriculture, Fisheries and Food, Fisheries Laboratory, Conwy, UK, which is suitable for all of the commonly cultured species, is given in Table 5. Note that diatoms require the addition of silica (Si) to the basic nutrients. The medium is then ready to dispense aseptically to the culture flasks, which are then ready to be inoculated. In recent years, several proprietary brands of algal culture nutrients have become available. These are generally based on the Guillard F/2 formula and provide excellent growth results (see Tables 3 and 4 for the basic formulae).
To obtain the maximum productivity of most species it may be necessary to dilute the seawater with pure (distilled) freshwater (or from an uncontaminated source) before filtration or autoclaving. Growth and cell division rates of Chaetoceros calcitrans, Thalassiosira pseudonana and Skeletonema costatum are optimal at a salinity of about 20 to 25 PSU. Productivity of many of the flagellates is optimal at 25 to 30 PSU.
Illumination for culture growth is provided by fluorescent lamps, usually mounted externally to the culture flasks (see Figure 18). The number of lamps used is determined by the height and diameter of the culture vessels with the object of providing 15 000 to 25 000 lux measured at the centre of the empty culture container. Two 65 or 80 W lamps are sufficient to illuminate 3 l glass flasks, which are about 18 cm diameter, whereas 5 lamps of the same light output are necessary for vessels of about 25 l volume (35 cm diameter). Growth is optimal at 18 to 22°C for most species.
Table 5: Nutrient salt stock solutions for the enrichment of diatom cultures in treated seawater. The addition of stock solution C is omitted in the culture of flagellates.

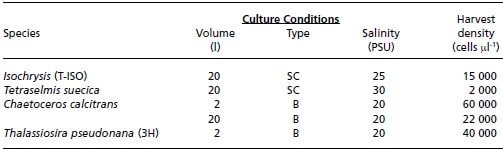
Examples of cell density achieved in the small-scale culture of a number of nutritionally important species are given in Table 6. These are values obtained at the MAFF Fisheries Laboratory, Conwy, and are typical of densities achieved elsewhere in commercial culture enterprises. It is interesting to note that much higher cell densities of Chaetoceros calcitrans are obtained in 2 l than in 20 l cultures. This does not necessarily mean that productivity in terms of biomass is lower. In all cultured species the size of cells is variable according to culture conditions and the growth phase. In 2 l cultures of Chaetoceros higher cell densities are reached but the individual cells are smaller: 35 ?m3 compared with 50 ?m3 in 20 l cultures. The dry weight content is also lower at about 10 ?g per million cells (micrograms per million cells) compared with up to 18 ?g per million cells in 20 l cultures. Other species show similar variability in size related parameters depending on cell density and conditions, quite apart from the inherent differences in cell size between species.
Through manipulation of culture conditions of the larger species, such as Tetraselmis, it is feasible to alter cell size so that the cells can be more readily ingested by smaller larvae. Small-scale culture systems can be technically improved to increase their performance by operating them as chemostats. But, if the objective is solely to produce more food, the better solution is to turn to large-scale culture methods.
Table 6: Cell densities at harvest (cells ?l-1) achieved in small-scale batch (B) and semi-continuous (SC) 2 l or 20 l cultures for a selection of nutritionally valuable species. The salinity of the culture medium is also given.