3.3.3 Estimating algal density
Before discussion of large-scale culture methods, a short description relating to the estimation of the density of cells in cultures at any scale is warranted. Various methods are available to estimate algal cell density including the use of spectrophotometers, fluorometers, haemocytometers, and Coulter counters (“multisizers”).
A spectrophotometer or fluorometer measures the chlorophyll A content in an algal culture and this can be used to obtain a quick approximation of cell density. Graphs comparing cell density and readings on either instrument must be prepared for each algal species. However, the chlorophyll A content in an algal cell is not constant and varies with nutritional state of the cell. This will affect the accuracy of cell density estimates derived when using these instruments.
More accurate estimates of cell density can be made using a haemocytometer or a Coulter Counter.
Haemocytometers are thick glass slides with two chambers on the upper surface, each measuring 1.0 x 1.0 mm. A special coverslip is placed over these two chambers giving a depth of 0.1 mm making the total volume of each chamber 0.1 mm3.
The base of each chamber is marked with a grid to aid in counting cells within the area (Figure 20). Prior to counting motile algal species, 1 or 2 drops of 10% formalin should be added to a 10 to 20 ml sample of the culture to be counted. With the coverslip in position, one or two drops of the algal sample are introduced by means of a Pasteur
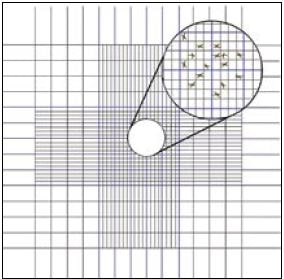
Figure 20: Diagram of the grid marked on a haemocytometer slide.
pipette to fill both chambers.
Cell density is estimated as follows. The central grid of each chamber (outlined in blue in Figure 20) is sub-divided into 25 squares (also outlined in blue in the diagram), each measuring 0.2 x 0.2 mm. Each larger square is further sub-divided into 16 smaller squares measuring 0.05 x 0.05 mm. The numbers of cells in 10 randomly chosen 0.2 x 0.2 mm squares are counted and the average or mean is calculated. This gives the mean number of algal cells per 0.2mm x 0.2mm x 0.1mm, or 0.004 mm3.
Example:
A. Counts of algal cells: 40 + 30 + 50 + 60 + 55 + 65 + 70 + 45 + 40 + 70 = 525
Average = 52.5 cells per 0.004 mm3
B. Multiply the average by 250 to give the average number of cells per mm3.
C. Since there are 1000 mm3 in 1 ml, multiply the value calculated in B by 1 000.
In this example, the cell density would be 52.5 x 250 x 1 000 = 13.1 million (13.1 x 106) cells per ml.
Note: 1 cell per ml (cells ml-1) = 1 000 cells per ?l (cells ?l-1)
An easier and more accurate method of estimating cell density is to use a Coulter Counter (now called a “multisizer” – see Figure 21). This instrument was developed primarily for counting blood cells.
Several models are available and all operate on the same principle. A small electrical current is passed between two electrodes. Each time a cell passes between them, the current is impeded and the cell is counted. The size of the aperture tube is important, and for counting algal cells of between 2 to 10 ?m an aperture of 50 or 100 ?m in diameter is required. A known volume of water is drawn through the opening in the aperture tube and the cells are counted. More detailed explanations of the operation of a Coulter Counter are available and are included in the suggested reading material at the end of this section.
Since algal cultures are often dense, samples must be diluted to a density that can be counted accurately when using an electronic counter – approximately 50 000 cells per ml (50 cells per ?l). Algal samples are usually diluted with a 3% solution of sodium chloride (by dissolving table salt in distilled water) or with 0.45 ?m membrane filtered seawater.
Example:
Add 0.2 ml of algal culture to 20 ml 3% NaCl. Mix well.
Count 3 times and obtain a mean value. Individual counts = 5 280; 5 336; 5 120.
If the volume of the solution sampled by the Coulter counter is 0.1ml, then the average = 5 245 cells per 0.1 ml.
Multiply 5 245 by 10 to obtain the number of cells in 1 ml of the sample, and multiply by 100 to correct for the dilution factor.
In this example, cell density would be 5 245 x 10 x 100 = 5.2 million (5.2 x 106) cells per ml.

Figure 21: Electronic particle counters used in hatcheries for counting the density of cells in algal cultures. A – a Coulter Counter; B – a Beckman Multisizer; C – details of the sample chamber of a Coulter Counter showing the aperture tube inserted in a sample container.
Electronic counters and particle sizers are expensive but used machines can be purchased for a reasonable price. The cost of purchase is quickly offset by the time saved and the accuracy of the counts.