4.1 BROODSTOCK CONDITIONING
4.1.1 Introduction
Conditioning broodstock is essential in the provision of larvae for culture (Figure 32). It is the procedure by which hatcheries are able to extend their production season, removing reliance on the relatively brief period in the year when adults of the desired species are bearing mature gametes in the sea. In the case of hatcheries in marginal climates, there is distinct advantage in producing seed early in the year – often months before stock have developed and matured in nature.
Early season production in colder climates ensures that seed have a maximum growing period prior to their first over-wintering. Thereby, they are larger and more resistant to low temperature. This may be relevant in the culture of exotic species where small seed may not be as fully cold hardy at a small size as are similarly sized seed of native species. Hatchery conditioning of stock is also relevant in circumstances where an exotic species has been introduced for farming purposes but will not recruit reliably in its new habitat.

Figure 32:
A typical broodstock conditioning system.
Many bivalves will mature in their first year of life as males. As they age, year by year, an increasing percentage may switch sex and become females.
This is known as protandric hermaphroditism. Among the commonly cultured species in hatcheries exhibiting this form of sexual development are clams of the genus Tapes, Mercenaria, Mya and Spisula, oysters of the genus Crassostrea and the many types of mussel including Mytilus sp. and Perna sp.
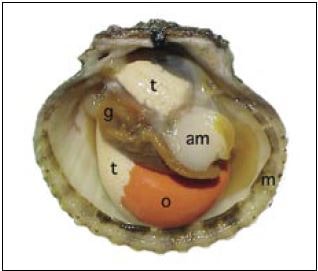
Figure 33: The anatomy of a fully mature calico scallop (Argopecten gibbus): am – adductor muscle; g – gills (raised to reveal the gonad); m – mantle; o – ovary; t – testis.
Some species of bivalves are truly functional hermaphrodites. They mature both male and female gonads simultaneously (Figure 33). Gametes are spawned sequentially, usually sperm first followed by the eggs, with later reversal to sperm again within the spawning cycle. This group of monoecious species includes the northern European King scallop, Pecten maximus, the (Brazilian or Caribbean) sand scallop, Pecten (Euvola) ziczac, the bay scallop, Argopecten irradians, the calico scallop, Argopecten gibbus, the Chilean scallop, Argopecten purpuratus, and some species of Chlamys. Sexes are separate (dioecious) in other large sea scallops, e.g. Placopecten magellanicus and Patinopecten yessoensis.
Flat oysters of the genera Ostrea and Tiostrea exhibit alternate sexuality. They switch sex at the end of each reproductive cycle. A single European oyster (Ostrea edulis) can go through two or three sex reversals each spawning season when sufficient food is available and during an extended warm water period.
Conditioning case history - the Manila clam, Tapes philippinarum

Figure 34: A selection of clams commonly cultured in hatcheries. Note that the nomenclature of the genus Tapes is synonymous with Venerupis and Ruditapes in European hatcheries, thus Manila clams may be referred to as Tapes or Venerupis or Ruditapes philippinarum (with semidecussatus or semidecussata being other alternative specific names). Nomenclature is equally as confusing in some other commonly cultured bivalves).
In the Manila clam (Figure 34), as in other bivalves, egg production increases with increasing adult size. Mature females of 10–20 g live weight will spawn 5–8 million eggs on average depending on their condition and time of year they are brought into breeding condition.
Populations of 2 and 3-year-olds show close to a 50:50 sex ratio. For example, of 138 conditioned clams subjected to spawning stimuli in trials at the MAFF Fisheries Laboratory, Conwy, UK, in 1987, 54 spawned as females and 55 as males. The remaining 29 clams, that were mainly in earlier season spawning attempts, failed to liberate gametes and were probably ‘under-ripe.’
Sexual development starts in the sea when the water temperature exceeds 10°C. Gametes develop during late May or June and mature in July or August to be retained until spawning is stimulated by high temperatures (>20°C) or by a series of thermal or handling shocks. In northern European waters, where temperatures are rarely high enough to stimulate spawning, mature gametes are retained into the early winter and are then resorbed.
Maturity can be accelerated in the hatchery by holding the clams at elevated temperatures and providing them with a suitable food ration. It is possible to mature adults in the winter and early spring, before clams in the sea commence sexual development, thereby extending the period hatcheries have access to larvae. Clams in spawning condition can, therefore, be made available for most of the year. To obtain spawning in the autumn it is possible to mature juveniles from early same season spawning by conditioning them at high temperature and high food rations.
Temperate climate bivalves generally have two spawning periods within a year following spring and autumnal peaks in phytoplankton production. Tropical species exhibit less well defined spawning periods. Spawning takes place during most of the year with a low percentage of adults maturing at any point in time. This habit presents problems for hatcheries in the tropics since many of the individuals will be spent (i.e., have recently spawned) or be in the early stages of gamete development when stock is brought into the hatchery. This is wasteful in terms of time, space and food resources. However, there are ways of bringing broodstock into greater synchronicity of reproductive development (see section 4.1.3).