4.1.2 Conditioning methods
4.1.2.1 Tank systems and water treatment
The basic methods for broodstock conditioning are much the same for all bivalves. It is usual for a hatchery to maintain its own stocks for production purposes in local, sea-based growout. These stocks are kept in the best possible conditions of high water flow and at low density in well maintained growout equipment. They are often the offspring of previous hatchery-reared generations, selected for desirable characteristics such as growth rate, shell shape and colouration.
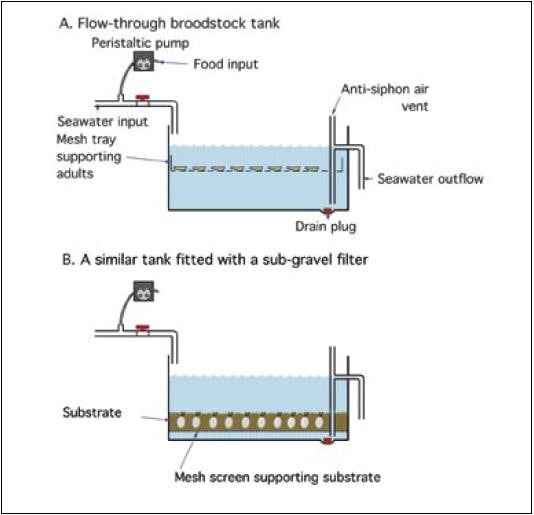
Figure 35: Diagrammatic representation of A – a flow-through broodstock tank in which adults are suspended off the bottom in a mesh tray with large apertures in the base so as not to retain faeces and detritus; B – a similar tank fitted for sub-gravel filtration. Systems of type A are suitable for most species that do not require a substrate. Clams and some scallop species often condition better in tanks of type B.
Adults taken from the sea are brought into the hatchery, their shells thoroughly scrubbed and rinsed to remove epifaunal (fouling) organisms and sediment, and then placed in tanks similar to those shown in Figure 35 (see also Figure 32). Clams and also scallop species (e.g. Pecten ziczac) which are normally partially buried in the substrate in nature, feed more efficiently if they are kept in a suitable substrate. In tanks of the type illustrated, clams or scallops are allowed to bury in 10 cm deep trays filled with coarse sand or shell gravel, or in a sufficient depth of substrate over a sub-sand filter (Figure 35B). Trays are supported off the bottom of the conditioning tank when stocked with bivalves that do not require a substrate, e.g. oysters, mussels and some scallop species (Figures 35A and 36).
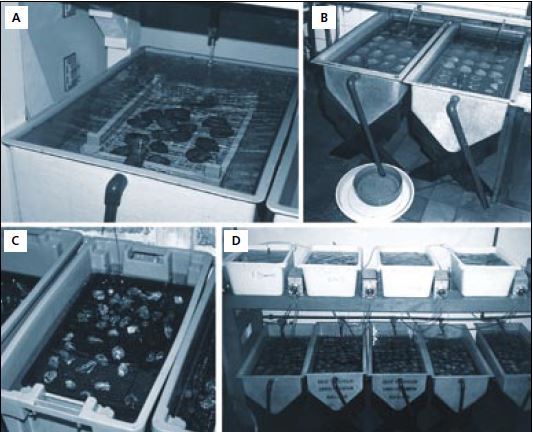
Figure 36: A to D – Examples of various types of flow-through tanks used for broodstock conditioning. The tray under the outflow in B – contains a mesh-based sieve, used to retain European flat oyster larvae that may otherwise be lost in the tank discharge when liberated by the adults. C – is an experimental system with each broodstock tank supplied a different diet by peristaltic pump from the reservoir tanks alongside.
Seawater used need not be filtered: the diversity of food species present in unfiltered seawater is beneficial in the conditioning process. While it is possible that the broodstock may be exposed to the infective stages of parasites or potentially pathogenic micro-organisms present in the incoming water supply, the cost benefit in not filtering the water often outweighs the risks. In most cases, conditioning takes place in flow
through systems, which may or may not include an element of water recycling to conserve cultured algae added as feed.
It is also feasible to condition bivalves in recirculation systems where the total live weight biomass of adults (the collective weight – shells included – of all the animals in the tank) does not exceed 2 or 3 g per l. In this case, it is advisable to drain and refill the total volume of water in the system at least twice each week to prevent build-ups of bacteria and metabolites.
Both salinity and temperature should be appropriate to the species being conditioned. Most commonly cultured bivalves will undergo reproductive development and mature gametes at salinities greater than 25 PSU (practical salinity units, equivalent to parts per thousand) and temperatures of between 16 and 24°C. However, each species will have optima for both of these parameters. Manila clams and Pacific oysters for example, respond best to water temperatures between 22 and 24°C. Pacific oysters will condition at a wide range of salinities (15 to 34 PSU) while Manila clams prefer higher salinities of between 25 and 34 PSU with an optimum of around 30 PSU. The American (Virginia) oyster, Crassostrea virginica, will condition at much lower salinities. As one would expect, offshore, deeper water species require cooler conditions and near oceanic salinity.
Water flow rate through conditioning tanks should exceed 25 ml per minute per adult and no more than 5 kg live weight biomass of stock should be held in a tank of 120 to 150 l volume (Figure 37). The water should not be recycled and reused in such small tanks when heavily stocked. When bivalves from outside the immediate area are used as stock, the effluent water discharged from the tanks should be diverted to a treatment tank to prevent the transfer of pathogens and parasites to the local environment. The effluent needs to be treated with >100 mg per l free-chlorine or a similarly effective disinfecting/sterilizing agent (e.g. ozone) for a minimum 24-hour period (preferably 48 hours) before it is returned to the sea.

Figure 37: A 120 l brood stock tank stocked with 55 oysters averaging 80 g live weight. The minimum flow rate of cultured food supplemented seawater through the tank at this stock density is 1.375 l per minute.
Hatcheries usually have a separate broodstock conditioning room or locate the conditioning tanks in a quiet area of the plant where stock will not be subject to frequent disturbance. Most species respond to shadows and vibration by closing their shell valves. The less disturbance they receive, the more time they will spend feeding.
Small and medium size hatcheries usually have between 5 and 20 conditioning tanks to accommodate the various species reared and to permit the regular introduction of new stock to maintain a rotation and ensure a continuous supply of larvae. Large hatcheries may have more of the smaller tanks or fewer that are larger. When the steady production of spat of a particular species is required over an extended period of the year, new stock is brought in to start the conditioning process on a weekly or two
weekly basis. In this way, adults are available for spawning every week.
4.1.2.2 Feeding broodstock
Cultured marine algal species are used most frequently as the principal food supply during conditioning. Alternative sources are natural phytoplankton bloomed extensively in outdoor tanks or ponds, or commercially available algae pastes.
Useful algal species that can be cultured intensively on a large-scale are Tetraselmis (various species, including T. chuii, T. tetrahele and T. suecica), Isochrysis galbana (and the T-Iso clone), Pavlova lutherii, Chaetoceros muelleri (previously named C. gracilis), Thalassiosira pseudonana and T. weisfloggii and Skeletonema costatum. (This list is by no means exhaustive). A mixture of these species, on a proportional basis, is more beneficial than a single species diet. Care should be taken not to feed relatively indigestible species (e.g. Chlorella sp.) or, species known to be deficient in the more highly unsaturated fatty acids (e.g. Dunaliella tertiolecta).
An example of the consequences of feeding a deficient diet is the reduced production of larvae from Ostrea edulis adults when held in filtered water and fed only Dunaliella tertiolecta (Table 8). Dunaliella is known to be lacking in the C20 and C22 highly unsaturated fatty acids considered to be nutritionally essential. In this trial, groups of 60 adults were kept in tanks provided with a through-flow of either unfiltered seawater or seawater filtered to 2 ?m particle size. (The experimental tank system is shown in the bottom right photograph of Figure 36C). A daily 3% ration based on the initial dry meat weight of the oysters was provided to three of the groups as Dunaliella alone or in combination with either Tetraselmis suecica or T-Iso. Control groups were kept in both flowing filtered and unfiltered seawater without the addition of cultured algae.
Table 8. Effect of diet on the production of Ostrea edulis larvae. Key: Seawater (SW) treatment, F and UF refer to filtered and unfiltered seawater respectively; Diet, Dt – Dunaliella tertiolecta, Ts – Tetraselmis suecica, T-Iso – Isochrysis galbana (Clone T-ISO). Days - refers to the number of days from the start of conditioning until larvae were first released. Total larvae is the number of larvae produced by each group of adults in a 70-day period and when this value is divided by the number of adults in the group provides larvae/oyster. From Millican and Helm (1994). See text for further details.
9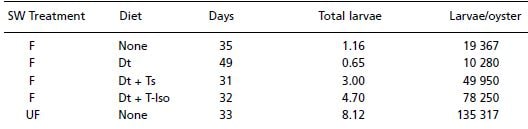
The elapsed time from the beginning of conditioning to the first release of larvae in each group was noted and daily counts of larval released were made during the 10- week duration of the trial. Results given in Table 8 show that the single species diet of Dunaliella delayed both the onset of larval production and reduced overall production in comparison with the alternative treatments tested. Interestingly, considerably greater numbers of larvae were released by adult oysters held in unfiltered seawater without cultured algae addition than from the other treatments. This reinforces the previously made point that there may be a cost benefit in not filtering seawater for conditioning.
The duration of the above trial encompassed the spring phytoplankton bloom when chlorophyll a in the unfiltered control seawater averaged 1.68 mg per m3 compared with 0.35 mg per m3 in the filtered control seawater. Particulate lipid averaged 62 ng per l (nanogram per l) compared with 9.7 ng per l respectively.
Methods for both intensive and extensive algal culture are described in Part 3 of this manual. Steps in the calculation of the required food ration are described below in section 4.1.2.3. The calculation does not, however, apply to extensively grown phytoplankton where species diversity, abundance and overall nutritional value will vary day by day. In this case, an approximation of abundance can be made by determining the ash-free dry weight of particulate matter per unit volume, or by organic carbon analysis. Alternatively, the operator can dilute the bloomed water “by eye” to provide an adequate ration.
Algal pastes of the various nutritionally preferred species are convenient to use and suppliers provide information on the equivalent number of cells per unit volume of the product. Many of these products also bear quantitative details of nutritionally important components on the packaging. Once opened, the non-living product has a relatively long shelf life when the supplier’s instructions are closely followed. Such pastes are probably best used in flow-through conditioning and attention must be paid to the hygiene of the tanks.
Provision of a satisfactory ration of nutritionally valuable food species during conditioning has a marked beneficial effect on egg production.
4.1.2.3 Calculating food ration for conditioning
The required food ration for conditioning is based on the dry meat weight of the adults. It is usually between 2 and 4% of the mean dry meat weight of the adults at the start of the conditioning period in dry weight of algae fed per day. Rations exceeding 6% are not conducive to successful conditioning. Rather, the bivalves will grow strongly in response to high feed levels and the high temperature of conditioning at the expense of reproductive development.
It is a simple procedure to determine the dry meat weight of a stock of bivalves of known live weight brought to the hatchery for conditioning. Opening a random sample of 10 or 12 individuals, removing the soft body tissues and weighing the meats after drying them to constant weight in an oven (60 to 80oC for 48 to 72 hours) will provide data for the calculation of ration. The equation below is to determine the dry weight of algae per adult required for a 3% daily ration.
Ration g per day per adult = 3 x mean dry meat weight (g)/100
Thus, a 3% ration for an adult of 0.75 g dry meat weight amounts to 0.0225 g dry weight of algae per day. Reference to the dry weight data given for the various algae species (see Table 1 – Part 3.1) shows that 1 million Tetraselmis cells have a dry (organic) weight of about 0.2 mg.
Assuming that 50% of the 3% daily ration (= 1.5%) is to be provided to the broodstock as Tetraselmis and the total dry meat weight biomass of the stock is 50 g (converted to mg in the equation below), then:
Ration (1.5%) per day (in millions of cells) = [(1.5x(50x1 000))/100]/0.2 = 3 750 million cells
If, for example, the harvest density of Tetraselmis on a particular day is 1.5 million cells per ml, then the volume required to feed the stock a 1.5% ration will be 3 750/1.5 = 2 500 ml, or 2.5 l. Calculation of the remainder of the ration is similar for the other component species of the diet. If instead of, or in addition to Tetraselmis, Chaetoceros muelleri is to be fed at a harvest density of 7 million cells per ml, then the volume required for a 1.5% ration will be 3.57 l. Chaetoceros muelleri has a dry weight approximating to 0.03 mg per million cells.
4.1.2.4 Adjusting ration for flow-through systems
In calculating ration, account needs to be taken of the configuration of the tanks and system in which the adults are conditioned. This is not of particular concern in closed systems where algal cells, as yet uneaten, are not lost other than through sedimentation or settling on surfaces. In flow-through systems and tanks of the type shown in Figures 32, 36 and 37, however, a proportion of the algae fed will inevitably be uneaten and will be lost in the outflow. For this reason, adequately stocked tanks of 100 to 150 l with slow rates of water exchange are preferred.
From experience, a total water exchange rate in excess of 90 minutes minimizes cultured algae loss, giving the stock adequate time to filter and consume 60 to 80% of the food. For example, a tank of 150 l volume stocked with 50 oysters or scallops of 75 to 100 g live weight requires a flow of 1.25 l per minute at 25 ml per minute per adult. At this rate of flow, tank volume exchange rate is 120 minutes. Where smaller bivalves, eg. Manila clams, are stocked, numbers of adults per tank should be increased correspondingly on a live weight biomass basis.
It is also preferred that the ration is dosed continuously into the water delivery line to the tank by peristaltic pump to effect better mixing. In some hatcheries, the daily food ration is divided into several batch feeds. The seawater supply is turned off for an hour or so after each addition. This can be problematic in terms of contamination with the waste products of metabolism if the water is inadvertently not switched back on in good time.
In the absence of the means to determine percentage particle removal between the in flow and out-flow from a flow-through tank, it is recommended that food supply be calculated as a 4% ration to allow for losses discussed above. If the operator has access to an electronic particle counter and sizer (e.g. a Coulter Counter – refer to Figure 21), adjustments to ration can be made based on hard data.
4.1.2.5 Two-stage early season conditioning
Conditioning can be a two-part procedure. Early in the season in temperate and cold-water climes, when adults in the wild have yet to start gamete development, it is often advantageous to provide conditions of high food availability at a temperature intermediate between the ambient and that required for conditioning. The objective is to boost the levels of food reserves in the adults that will later be mobilized in gamete development. This is more important for females than it is for males because egg development and maturation is considerably more energy intensive. Following 4 to 6 weeks of a high ration: moderate temperature regime, temperature is gradually raised (1 to 2°C per day) and food ration is somewhat reduced (from 4 to 6% to 2 to 3% per day).
Food supply for the first stage, which can be called the pre-conditioning stage, can be in the form of algal pastes, bloomed natural phytoplankton (from extensive algal culture, Part 3.4.6) or, intensively cultured algal species. It is important to bear in mind that during this stage, principally the structural lipid (phospholipid) composition of the early stage oocytes will be influenced by the diet and ration available to the broodstock. Thus, a diet deficient in highly unsaturated fatty acids (HUFAs) of known importance, including EPA (eicosapentaenoic acid, 20:5n-3) and DHA (docosahexaenoic acid, 20:6n-3), will be reflected in eggs with cell membranes with reduced content of these components. For this reason, the ration should contain nutritionally valuable diatoms (e.g. Chaetoceros muelleri or Thalassiosira sp.) and flagellates such as Pavlova lutherii or Isochrysis galbana, all of which are rich in one or other of these HUFAs.
Triacylglycerols – the neutral lipids that are laid down as reserves in maturing eggs – are accumulated during the later stages of the second, warm water phase of conditioning. These lipids are drawn upon as sources of energy during embryo and larval development. Their composition appears to be more dependent upon lipids being mobilized directly from the food ingested by the adult than it is from maternally derived reserves.