4.2.3 The special case of flat oysters
Before considering spawning in clams, scallops and mussels the special case needs to be noted concerning oysters of the genera Ostrea and Tiostrea. These, unlike other commonly cultured bivalves, do not need to be stimulated to spawn. They will spawn of their own accord during the conditioning process and will brood larvae within their mantle cavities for varying periods of time depending on species and temperature.
This group of oysters, including the European flat (or “Belon”) oyster, Ostrea edulis
(Figure 40), the New Zealand (“Bluff” or mud) oyster, Tiostrea lutaria, and the closely related Chilean flat oyster, Tiostrea chilensis, are referred to as larviparous. The latter two species release their larvae into the surrounding water after about a

Figure 40: Anatomy of a developing flat oyster, Ostrea edulis; am – adductor muscle; g – gonad tissue overlying the digestive gland; gl – gills; h – hinge; ic – inhalant chamber of mantle cavity.
At spawning, eggs pass through the gills into the inhalant chamber of the mantle cavity where they develop to fully shelled larvae over the course of a week or more, depending on species. The parent releases larvae when they are able to ingest and digest algae. (The anatomy of oysters of the genera Tiostrea and ostrea is essentially similar).
20-day brooding period when the larvae are between 450 and 490 ?m shell length and are almost ready to set. In contrast, the European flat oyster releases its larvae, after a brooding period of 6 to 8 days at normal conditioning temperatures, when they are 170 to 190 ?m shell length and require a further 10 to 12 days of culture before they reach maturity and are ready to set. Eggs of the New Zealand and Chilean flat oyster are 350 ?m diameter compared with 150 ?m in the European flat oyster.

Figure 41: Brooding stages of the European flat oyster, Ostrea edulis. W – the “white sick” stage shortly after eggs are passed to the inhalant chamber of the mantle cavity; G – the “grey sick” stage, beyond the trochophore stage, when the shell valves are well developed but the larval organs not yet fully developed (3 to 5 days after spawning); B – the “black sick” stage at which larvae are almost fully developed and are ready to be released. White, grey and black “sick” are traditional terms applied to brooding oysters in Europe.
The above species are not mass spawners. Rather, stocks of adults produce larvae over an extended period. It is extremely rare to see mature males liberating sperm into the surrounding water and it is assumed that they do so periodically in small quantity. Adjacent female-phase oysters (these species exhibit alternate sexuality) draw in sperm in their inhalant current, in the same way as food particles, and in response, release their eggs into the exhalant chamber of the mantle cavity – as do oviparous species. However, the eggs are not expelled into the surrounding water. Instead they are passed through the gill filaments into the inhalant chamber of the mantle cavity where they are fertilized and develop over an extended period (Figure 41), to be fully motile, completely shelled veligers at the time of release (Figure 42).
Hatchery technicians experienced in rearing these species, can often identify spawning and brooding female-phase oysters from small quantities of eggs that escape mantle cavity retention and settle on the upper shell valve, adjacent to either the inhalant or exhalant mantle apertures. Brooding oysters also tend to be inactive, retaining only a minimal shell gape for long periods.
When larvae of the larviparous oysters are liberated into the water they either swim to the surface forming visible “rafts” in the case of O. edulis, or they
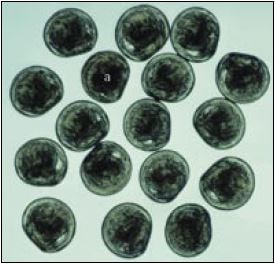
Figure 42: The appearance of Ostrea edulis veliger larvae (175 ?m mean shell length) at release from the adult. All larvae are normally formed except for - a - which exhibits incomplete development of one shell valve.
immediately seek a surface upon which to settle and undergo metamorphosis in the Tiostrea sp. In the latter case, suitable settlement surfaces need to be added to the broodstock tanks in advance of larvae liberation. The surfaces can either be shell or plastic cultch materials or plastic mesh (see later section dealing with settlement).
When the expected liberation period is reached in the case of O. edulis, tanks should be checked every 2 or 3 hours for signs of larval release. Swimming larvae can be skimmed from the water surface of the conditioning tanks with a beaker or a small 90 ?m mesh sieve and transferred to a bucket of water. Alternatively, they can be allowed to flow in the tank discharge into a larger sieve of the same mesh aperture, which is partially submerged in a tray of water (Figure 43). It is always best to collect the larvae as soon after release as possible to avoid larvae becoming contaminated by adult faecal matter in the water, or being filtered out of the water by the filtration activities of the adults.

Figure 43: Experimental broodstock conditioning of Ostrea edulis. Note the green coloured sieves immersed in shallow trays to catch and retain larvae.
Once a brood has been collected they are counted (see later) and distributed between culture tanks at the appropriate density. Female-phase European flat oysters of 70 to 90 g (the size of oysters in Figure 41) will liberate broods of between 1 and 2.5 million larvae. In contrast, female-phase Tiostrea oysters, which produce considerably larger eggs, will liberate much smaller broods of 20 000 to 50 000 larvae.
Larvae can be removed from adults identified as brooding either from the conditioning tanks, or in stock brought back from growout – or from wild populations – during the natural breeding season. The steps in this procedure are illustrated in Figure 44. It is sometimes used as a method to obtain larvae before they have developed a functional gut in the later stages of brooding. This can be relevant in the summer when potentially pathogenic bacteria are prevalent. There is evidence to suggest that brooding larvae begin feeding while still in the parental mantle cavity and, thereby, may be exposed to high loads of bacteria and other micro-organisms accumulated and defaecated both by the parent and adjacent stock.
Whether larvae are liberated naturally by the stock or are removed prior to release, they are grown following the standard methodology described later in the larval culture sections of this manual. Best results are with broods that have developed to the fully shelled, motile, D-larva stage. If removed at an earlier developmental stage, food is withheld until larvae have developed a fully functional alimentary system – visible through the transparent shell valves as a darker S-shaped structure, which can be seen in Figure 42. This may take 2 or 3 days from the time of removal. Prior to this stage, the soft body tissues are a densely, granular, grey colour and the larvae only weakly motile (see Figure 41 – “grey sick” larvae).

Figure 44: A – Stripping Ostrea edulis larvae from a brooding adult.
B – The top (flat) shell valve is removed, then the brooding larvae are washed through a 90 ?m sieve balanced over a bucket of filtered sea water (C). D – Most of the larvae swim rapidly to the water surface where they aggregate (raft) together. They are then ready to be sam pled for counting and for size determination.
Photographs were taken at the Harwen Oyster Farm hatchery in Nova Scotia (courtesy John and Krista Harding).