5.2.3 Diet composition and ration
A suitable starter diet for D- and early-stage larvae (<125 ?m shell length) of most commonly cultured bivalves is a mixture of:
One of the following diatoms:
Chaetoceros calcitrans or Thalassiosira pseudonana (for larvae >55 ?m) or Chaetoceros muelleri (for larvae >90 ?m),
combined with:
One of the following flagellates:
Isochrysis galbana or ‘T-Iso’ or Pavlova lutheri, in equal proportion by cell numbers.
When the mean size of larvae exceeds 120 ?m shell length, the larger cell-size flagellate, Tetraselmis spp. (T. chuii, T. suecica, T. tetrahele, etc.), can usefully be added to the diet.
Food rations are usually quoted as the total number of algal cells per microlitre (cells per ?l) or per millilitre (cells per ml) of the culture tank volume. Note that 100 cells per ?l are equivalent to 100 000 cells per ml.
Account needs to be taken of the fact that the cells of the different algal species vary widely in mean size and, thereby, in volume and mass (see Table 1, Part 3.1). In calculating a ration for a diet incorporating two or three species, the representation of each in the ration is calculated on a cell volume equivalency basis, where (in approximate terms):
1.0 cell of Isochrysis galbana, T-Iso or Pavlova lutherii =
0.1 cells of Tetraselmis sp., or
1.0 cells of Thalassiosira pseudonana, or
2.25 cells of Chaetoceros calcitrans, or
0.75 cells of Chaetoceros muelleri
Thus, a suitable food ration for early-stage Crassostrea or Tapes larvae (and for most other species), where the target food cell density is equivalent to 100 cells Isochrysis per ?l, can be satisfied by the following dietary combinations:
125 cells per ?l C. calcitrans + 50 cells per ?l I. galbana, or
37.5 cells per ?l C. muelleri + 50 cells per ?l P. lutherii, or
50 cells per ?l T. pseudonana + 50 cells per ?l P. lutherii
Any of these mixed-species diets are excellent for larvae of bivalves most commonly cultured in hatcheries, although the ration as cells per ?l will vary both with species and density of larvae in the culture. Cell densities quoted above are ideal for larvae of the various Crassostrea sp., Ostrea edulis, the clams Tapes philippinarum, Tapes decussatus, Mercenaria mercenaria, Mya arenaria (and many others), and the mussels Mytilus edulis and Perna perna at previously quoted larval densities (refer to Table 10, Part 5.1.2.3). In contrast, larvae of many scallop species show better overall performance when fed the same diets but at lower rations. For example, larvae of the scallops, Pecten ziczac and Argopecten gibbus, express maximum growth rates at a total ration of between 5 cells per ?l at the D-larva stage, increasing to 18 cells per ?l prior to the pediveliger stage. Other hatcheries use rations two to three times greater with larvae of different scallops, but rarely do they use rations as high as for oysters, clams and mussels, over a similar range of larval sizes.
It will be noted in the example of dietary combinations given above that T-Iso has been omitted. While T-Iso is a perfectly good species to feed to larvae of clams, mussels and scallops, there are reservations to its use in diets for the early larvae of Crassostrea species (Figure 70). Compared with Isochrysis galbana and, more so, Pavlova lutherii, levels of the important highly unsaturated fatty acid (HUFA) DHA are considerably lower. When T-Iso is fed as a single species diet to larvae of the various Crassostrea sp., both larval growth and development are severely retarded beyond a shell length of 110 ?m. For this reason, it is recommended that hatcheries should concentrate their small flagellate, algal culture on well proven strains of Isochrysis galbana and Pavlova lutherii.
Larvae can be grown from D-larvae to metamorphosis on two-species combination diets, such as those shown above. However, once mean larval shell length exceeds 120 ?m, it is advantageous to add a third species in the form of one of the smaller Tetraselmis sp. Evidence shows that growth rate and the proportion of larvae that successfully complete metamorphosis improves when Tetraselmis is included in the diet (refer to Figure 68).
Either Tetraselmis can be used as a direct substitute for Isochrysis or Pavlova in the diet or, better still, it can be used as an additional species in formulating a three-species diet. It should not, however, be substituted for the diatom in the diet. Each of the three recommended diatoms, mentioned above, contains another important HUFA (EPA) of known nutritional and developmental significance.
When substituted for Isochrysis or Pavlova in a two-species diet, Tetraselmis is fed at 10% of the cell density appropriate to the smaller flagellates, thus:
37.5 cells per ? l C. muelleri + 50 cells per ?l P. lutherii
becomes:-
37.5 cells per ? l C. muelleri + 0.5 cells per ?l T. suecica
When used as an additional species to make a three-species combination, each of the component species is provided at 33.3% of the target cell density, which may be 100 cells per ? l Isochrysis equivalents. Thus:
Two-species combination:
37.5 cells per ? l C. muelleri + 50 cells per ?l P. lutherii =
100 cells per ?l Isochrysis equivalents;
Three-species combination: 25 cells per ?l C. muelleri + 33.3 cells per ?l P. lutherii + 3.33 cells per ?l T. suecica =
100 cells per ? l Isochrysis equivalents
The overall amount of algae fed in terms of cell volume/mass is approximately the same for these two example diets, both of which are suitable for larvae >120 ?m shell length.
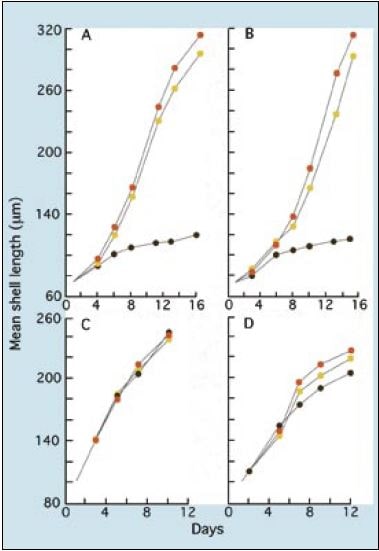
Figure 70: The growth of (A) Crassostrea gigas, (B) Crassostrea rhizophorae, (C) Mercenaria mercenaria and (D) Tapes philippinarum larvae fed T-Iso (brown circles), Chaetoceros calcitrans (yellow circles) and a two species mixture of these two algae (orange circles).
Attention so far in this section has focussed on general guidelines for diets and rations for larvae. In many small owner operator hatcheries run on the “wet thumb” principle – and often on a very tight budget – little or no regard is paid to counting the density of algae at harvest, or in formulating a combination of species to feed with any great degree of precision. An experienced operator rearing one or more of the hardy and tolerant bivalve species will judge which algae cultures look to be the best on a particular day and will add enough of each to the larvae tanks until the water colour looks about right.
At the other end of the spectrum – in large hatcheries supplying seed at industrial scale either for their own growout, or in support of private growers within a region – responsibility and the scale of financial investment dictate that husbandry is maintained under closer control. Here, the priority is to maximize cost-efficient output of the seed products and make a profit. How this can be achieved by making the most effective use of cultured algae in feeding larvae is discussed next.
5.2.3.1 Feeding strategies
There are two basic strategies used in hatcheries to ensure that a sufficient food ration is provided to larvae. The first is to add algae to the seawater volume contained in the larval culture tanks with the objective of raising food cell density to a concentration that supports maximum larval growth rate. The second strategy involves feeding the ever
increasing biomass of larvae, as development proceeds, according to known food-cell ingestion rates of larvae of different mean shell lengths. This latter approach has already been briefly mentioned in discussion of high density larval culture in Part 5.1.4.1.
Strategy 1 is the easier option, since the volume of water in a tank is unlikely to be varied during the culture period. It is the appropriate strategy where lower densities of larvae are maintained. In effect, the food ration is delivered once per day. Over the course of the next 24-hour period the food will be grazed to a low level. It is only for a brief period in the 24-hour period that food cell concentration is optimal. However, the strategy can be modified, and often is, by feeding an additional 50% (or more) ration 8 to 12 hours after the main ration. The intention is to maintain food cell density nearer the optimum for the greater part of the day. At low mortality rates, the numbers of larvae may need to be reduced as development proceeds by division between two or more tanks so that the ration is not grazed too quickly.
Strategy 2 requires knowledge of the rate at which food cells are depleted from the water by a known number of larvae at all shell lengths (or weights) for all stages in development from the D-larva stage to metamorphosis. Having determined mean size and the number of larvae surviving at successive tank water changes the operator can calculate how much feed needs to be added to the tank to support maximum growth rate for the biomass of larvae at that time. In this way, higher densities of larvae can successfully be maintained in a given tank volume.
However, over-feeding is equally if not more damaging to the performance of larvae than is under feeding. As mentioned previously, at higher larval densities it may be necessary to feed twice each day as two separate rations at the recommended optimum cell density for the larvae of most species, i.e. at close to 100 cells per ?l Isochrysis
equivalents. Providing a double ration as one bulk feed per day to a culture of larvae will exceed the density and volume of food cells at which feeding activity of larvae is at its most efficient. Overfeeding may lead to bacterially related disease in situations where larvae are already stressed. In this case, the required ration is divided into two equal parts. The first part is added directly to the tank and the remaining half is dosed or drip fed over the following 24-h period.
A logical development in ensuring proper feeding of larvae is the use of modern, sophisticated opto-electronics. Some progress has been made with more primitive devices that shine an infra-red light beam through a culture to a detector, comparing the turbidity caused by the presence of the optimal food cell density in the culture volume with a reference signal. When food cells are grazed by the larvae, turbidity of the water decreases. At a certain preset value, a relay is triggered which activates a small peristaltic pump that adds more algae to the tank from an aerated reservoir until the desired turbidity is restored. The reader is referred to Part 5.1.4 for further information.
5.2.3.2 Calculating food rations
Feeding Strategy 1: Volumes of the algal species necessary for addition to larval rearing vessels to achieve the required cell densities are calculated from the following equation:
Volume (l) to feed = required cell density [cells per ?l] x V
cell density of harvested algae [cells per ?l]
where V = volume of the larvae culture tank in litres.
Example:
Basic Information:
Diet and cell density to be fed:
37.5 cells per ?l C. muelleri + 50 cells per ?l P. lutherii
Cell densities of harvested algae:
C. muelleri 4 800 cells per ?l
P. lutherii 8 900 cells per ?l
Volume of larvae culture = 800 l
Calculation:
Volume of C. muelleri required = 37.5x800/4 800 = 6.25 l
Volume of P. lutherii required = 50.0x800/8 900 = 4.49 l
Feeding Strategy 2: Calculation involves determining the number of food cells additional to an initial daily ration equivalent to 75 cells per ?l Isochrysis required during the following 24 hours to maintain the algal cell density constant. Steps in the calculation are detailed in the following example that applies to Crassostrea gigas larvae:
Example:
Basic Information:
Larvae culture tank volume - 1 000 l
Number of C. gigas larvae - 22.5 million
Mean shell length of larvae - 170 ?m
Algal diet provided - equal mixture by cell volume
of: P. lutherii, C. muelleri, T. suecica
Harvest densities of algae - P. lutherii = 15 000 cells per ?l
C. muelleri = 7 400 cells per ?l
T. suecica = 1 200 cells per ?l
Calculation:
a) Provide an initial ration of 25 cells per ?l P. lutherii, 18.75 cells per ?l C. muelleri and 2.5 cells per ?l T. suecica = 1.67, 2.53 and 2.08 l respectively at the harvest cell densities given above (see Feeding Strategy 1 for the method of calculation).
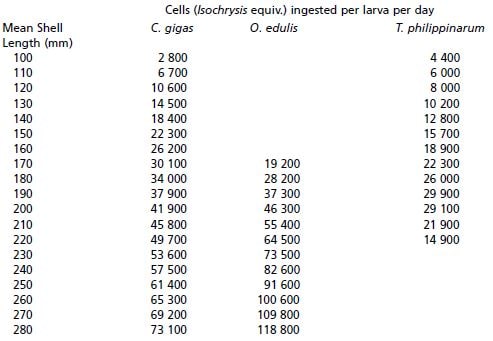
b) Read the number of cells consumed by a 170 ?m Pacific oyster larvae in 24 hours from Table 13 = 30 100
c) Divide 30 100 by the number of algae species in the diet = 10 033 cells per ?l Pavlova (1 003 cells in the case of Tetraselmis and 7 525 cells in the case of C. muelleri to account for cell volume differences).
d) Calculate the volume of harvested algae of each species required to maintain the optimum food cell density in the 1 000 l tank stocked with 22.5 million larvae:
Vol. of Pavlova = value in (c) x number of larvae (millions)
cell density of algal culture [cells per ?l]
= 10 033x22.5/15 000 = 15.04 l
Similarly, the volume of C. muelleri required is: 7 525x22.5/7 400 = 22.88 l and for T. suecica it is: 1 003x22.5/1 200 = 18.81 l
e) Add the volumes calculated in (a) above directly to the larval tank. The remainder (15.04 minus 1.67 l for Pavlova, etc.) is mixed in a cooled, aerated reservoir of sufficient volume. Dose this volume at a constant rate over the 24-hour period. From the practical point of view, it is advisable to make up the algae contained in the reservoir with filtered seawater to the volume pumped in 24 hours by the peristaltic pump.
Note: Data given in Table 13 applies to larvae grown at 24+1°C. At a fixed rearing temperature, the growth of larvae is generally predictable so that daily measurements of shell length are not essential. Measurements should, however, be made at 48-hour intervals and can be estimated on the intermediate days based on experience.
Table 13: The number of algal cells ingested per larva per day by three commonly cultured bivalves relative to the mean shell length of the larvae. Values are shown as cells equivalent in size to Isochrysis galbana.
Growth rates of larvae are not significantly different whether they are grown at low density using Feeding Strategy 1 or at high density using Feeding Strategy 2. The advantage of the latter strategy is in operational cost efficiency, both in terms of labour and the better use of hatchery space. When using opto-electronic control of feeding (as in Part 5.1.4.1) estimated volumes of the required food species are calculated as in Feeding Strategy 2.