5.4.3 Setting larvae
5.4.3.1 Settlement stimuli
Strategies to facilitate and enhance settlement of pediveligers vary widely among hatcheries according to species and to the methods that will be employed to grow the early juveniles. Hatchery managers want larvae to settle on a convenient substrate (cultch – see 5.4.3.2) and to begin metamorphose as quickly as possible.
Studies have shown that several methods, including both physical and chemical stimuli help initiate these processes. The most common physical method used is temperature shock, chilling mature larvae (sometimes in a refrigerator) and then placing them in warm water in setting tanks. Results have been variable but there is an indication that the success rate of metamorphosis can be improved when this method has been used.
A common method to stimulate and increase the success of metamorphosis is the use of chemicals. Several have been tried including ammonia and a group of chemicals known as neurotransmitters including L-DOPA (L-3-4-dihydroxyphenylalanine), epinephrine, norepinephrine and yohimbine.
Many hatchery managers question the use of chemicals to stimulate and increase the success of metamorphosis and they are not used at many hatcheries. Managers believe that high rates of successful metamorphosis of hatchery produced bivalve larvae can be attained at either a hatchery or a remote setting site if the larvae are of high quality with good food reserves and are handled properly. They believe addition of neurotransmitters may initially yield higher rates of successful metamorphosis than untreated larvae, but little if any difference is observed in the number of juveniles that grow to 5 to10 mm in treated or untreated larvae. Neurotransmitters have permitted some larvae to metamorphose that ordinarily would not have been able to do so but they do not have sufficient reserves to develop further into juveniles.
5.4.3.2 Suitable settlement substrates
The material used to settle larvae on at hatcheries or remote setting facilities is termed cultch and it can be a variety of materials. Two important criteria for cultch are that it must be a suitable surface for larvae to settle on and it must be easily handled.
Oyster hatcheries on the West Coast of North America do not always settle the pediveligers themselves but supply growers with eyed larvae to be set remotely at locations adjacent to the oyster farms (Figure 85). This methodology is dealt with in Part 6.2

Figure 85: A remote, oyster setting system located on Vancouver Island, British Columbia, Canada. Eyed Pacific oyster, Crassostrea gigas, larvae are received from West Coast hatcheries and are set in concrete tanks packed with net bags filled with clean, aged Pacific oyster shells. Once the shells have a sufficient set – a few days later – they are moved to nursery growout at the farm.
The following is a synopsis of the more common methods employed to set mature, eyed larvae of the various bivalve groups.
(i) Oysters
Surfaces are provided for settlement either in the tanks in which the larvae are grown – directly in the broodstock tanks in the special case of Tiostrea larvae – or in special-purpose settlement tanks. This is when 50% or more of the larvae are at the eyed stage and applies equally to Ostrea and Crassostrea species. Hatcheries will often grade out the largest larvae in a batch on a 240 ?m mesh (retaining larvae of 300 to 340 ?m shell length) for settlement, leaving the remainder to grow and develop further. Appropriate densities of oyster larvae per unit volume at settlement are within the range 2 000 to 5 000 per l although the area of settlement surface is the more important criterion. Types of materials in common usage to provide large surface areas for settlement include the following:
a) Sheets of slightly roughened PVC, which may be vertically stacked in the water column with each sheet separated by a spacer, or be a single sheet lying on the base of the tank. (PVC sheet formed in the shape of semi-cylindrical roof tiles are also sometimes used).
b) Layers of shell chips and particles prepared by grinding aged, clean oyster shell spread over the base of settlement trays or tanks. The particulate material is graded so that only pieces that pass through a 500 ?m but are retained by a 250 ?m screen are used.
c) Bundles, bags or strings of aged clean oyster shells dispersed throughout the water column, usually in settlement tanks.
d) Various plastic or ceramic materials coated with cement (lime/mortar mix). For example, stacks of cement-coated, plastic Chinese “hat” collectors are sometimes used to settle oyster spat in large tanks. Once grown to a suitable size spat can be removed by bending and flexing the collectors to break apart the cement coating.

Figure 86: A and B – In this example, matt surfaced PVC sheets used as settlement substrate for oyster spat are placed on the base of larval culture tanks (A). The tanks are illuminated by overhead, tungsten filament lamps to aid rapid settlement. Spat collectors are checked several times each day (B) and when the set density is sufficient, the newly settled spat are gently removed with a razor blade. C and D – Staff at a Cuban oyster hatchery stringing mangrove oyster shells on lengths of nylon twine (C). These strings are placed in concrete settlement tanks with sufficient eyed larvae to provide the required set density (D). Large-volume larval culture tanks can be seen behind the settlement tanks in photograph D.
Larvae tend to settle and attach more prolifically on the shaded under surface of substrate materials in shallow tanks. A low intensity tungsten filament lamp (60W), mounted above deeper tanks, will also encourage larvae to settle towards the bottom in the more shaded areas (Figure 86A and B). The attractiveness of large surface area collectors can be enhanced by painting them with an aqueous extract of homogenized oyster flesh. They are then allowed to air-dry before being placed in the settlement tanks. The reason is that larvae exhibit gregarious behaviour and will tend to attach where others have attached before. PVC collectors improve in their ability to attract settlement with usage over time. When sufficiently “aged” they do not require coating with the aqueous extract.
Methods (a) and (b) above are used to produce what are known as “cultchless” spat. Cultchless oyster spat (spat that are no longer attached to a substrate or are attached to a shell particle) can then be grown as separate individuals through to marketable size to service the half-shell trade. In contrast, survivors of those set on whole shells will eventually grow together, their shells fuse and form clusters, and are suitable only for meat extraction when harvested.
To provide cultchless spat when using PVC collector sheets, the newly set spat need to be removed from the surfaces with a razor blade within 24 hours of attachment. This is done by immersing the sheet in a shallow tray of seawater and gently scrapping the razor blade, mounted in a suitable holder, across the surface while spraying the blade with a jet of seawater. The number of removed spat can be estimated using the same method as for larvae (section 5.1.2.3). They are then moved to the within hatchery, early juvenile culture system.
As previously mentioned, eyed oyster larvae can be encouraged to undergo metamorphosis without cementing themselves to a substrate by the use of the neuro transmitter, epinephrine. This involves dissolving 0.1832 g of epinephrine (adrenalin) in a little 10% hydrochloric acid and then diluting it in 10 l of filtered seawater, which is a sufficient volume to treat 2 million eyed larvae. Larvae of settlement size are exposed to this treatment for 60-90 minutes and are then returned to the culture tanks. At the next water change, larvae that have metamorphosed and have begun growth as spat are graded from those that are still larvae by retaining them on a 270 ?m mesh sieve. Only larvae ready for imminent settlement will respond to this treatment and will complete metamorphosis without fixation. Larvae that do not respond are unharmed and can be treated again one or two days later. This method of treatment can be used with or without the provision of settlement surfaces (usually with).
Post-settlement survival rates in oysters are generally high with 50–70% of those that set reaching 2 mm shell length.
(ii) Scallops
In contrast to oyster larvae, eyed scallop pediveligers form a byssus attachment to surfaces upon which they settle. They will attach to filamentous red algae, hydrozoans, bryozoans and polychaete tubes in nature, amongst other suitable living and non-living substrates. Polyethylene netting, nylon mesh and a variety of other similarly filamentous materials provide satisfactory substitutes in the hatchery. Eyed pediveligers can be set in larval tanks or in purpose-provided settlement tanks, either in static water conditions or in flow-through. In the latter, a mesh screen on the out
flow is essential to retain larvae within the tank. Since scallop larvae, pediveligers and early juveniles are particularly fragile and delicate the tendency is to remove them from the larvae tanks when eyed and to transfer them to settlement tanks. This is at a considerably smaller shell length than for oyster larvae – 220 to 240 ?m compared with 300 to 340 ?m. Examples of suitable types of settlement tank and collector materials are shown in Figures 87 and 88.

Figure 87: Scallop pediveligers can be set at a density of up to 2 000 per l in cultch filled tanks equipped for static water, recirculation or flow-through. The system illustrated is at the Bermuda Biological Station of Research, Inc. and is used for both Argopecten gibbus and Pecten ziczac. See text for an explanation of the steps involved.
Scallop pediveligers can be set at densities of between 1 000 and 2 000 per l in cultch filled tanks equipped for static water, recirculation or flow-through. The example shown in Figure 87 utilizes circular, fibreglass fish tanks of 450 l volume (A) fitted with bottom drains and standpipes. Bundles of plastic mesh netting (B) are loosely packed in the tank(s) (C) or are enclosed in fine mesh “onion” bags suspended in the water column (D). Spat set mainly on the black mesh (E). The plastic pipe arrangement above the water surface, clearly visible in D, is part of an air-lift driven upwelling system. Each vertical limb has an air-line fitted at the base. With the air flow turned on, water is lifted from the base of the tank to be sprayed from drilled holes in the above-water delivery pipes and back into the tank. In operation the water level in the tank half covers the delivery pipes.
Settlement tanks are treated as larval culture tanks for the first 6 to 8 days once pediveligers have been added. Water is changed 3 times during this period by draining water through a sieve to retain the remaining swimming larvae (note the drain valves visible in Figure 87A). At the same time, filtered seawater is added at a rate to balance with the out-flow in order to maintain the water level constant, which prevents the cultch and attached larvae from being exposed to air. This water exchange is continued for 30 to 45 minutes. Numbers of larvae retained on the sieve, their survival and the numbers of metamorphosed but unattached spat are estimated before returning them to the tank. Tanks are gently aerated during this period and are supplied with food in exactly the same way as in larval culture.
After this first week, the air-lift driven upwelling system is switched on and the temperature of the water in the tank is gradually lowered over a period of days to the ambient. The tanks are then operated on flow-through by turning on a continuous and sufficient in-flow of ambient temperature seawater to exchange the tank volume 3 or 4 times each day. The air-lift driven upwelling is maintained and food is added continuously. Three weeks after introduction of the pediveligers the largest attached spat measure 2 mm shell height (Figure 87E).
In essence, the process described above is a hatchery adaptation of the widespread use of plastic mesh contained in “onion” bags to capture natural spat in the sea. A different approach is to set pediveligers in trays or cylinders with suitable aperture mesh bases (120 or 150 ?m aperture). The trays are held in shallow tanks through which food supplemented water is recirculated or flows continuously to waste (Figure 88).
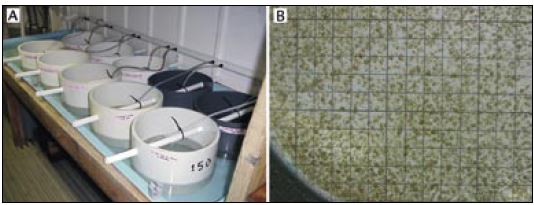
Figure 88: A – cylindrical, nylon mesh based trays used to set scallop pediveligers at the Bermuda Biological Station for Research, Inc. The trays are partially immersed in shallow raceway tanks through which seawater can either be recirculated or flow to waste. Each tray receives a downwelling flow of cultured food supplemented seawater. B – the appearance of 3-week old Argopecten gibbus spat growing attached to the mesh base of the tray. A grid marked in 1 cm squares has been placed beneath the mesh to indicate the density of set and enables estimates of numbers to be determined.
Pediveligers are stocked in the trays at a density no greater than 100 per cm2 of the base area. For example, a 25 cm internal diameter cylinder has a bottom mesh area of approximately 500 cm2 and can be stocked with up to 50 000 pediveligers. Space availability for settlement of the pediveligers and for the growth of those that attach and metamorphose to juveniles is critical in determining stock density. Spat are mobile and will respond to overcrowding by detaching their byssus attachment and swimming in search of a less crowded area to re-attach. Soft tissue damage resulting in mortality will occur if spat collide with their neighbours and interlock their shell valves.
Various adaptations of the concept of setting scallop pediveligers in shallow, mesh based trays are used in Europe for Pecten maximus.
Post-settlement survival rates in scallops are usually not very high; 15 to 30% from the initial number of pediveligers to 2 mm shell height is considered normal. Survival tends to be greater using the set in trays method (Figure 88) but growth rate is superior when using bundles or bags of mesh (Figure 87). This is probably because spatial separation of the attached spat is much improved over the large surface area provided throughout the volume of the tank in the latter case.
(iii) Clams and mussels
Clam larvae begin substrate search behaviour at a similar shell length to scallop larvae (at 220 to 240 ?m). They also attach to surfaces and to each other, with byssus threads. A convenient way to handle them at this stage is to transfer them to a settlement tank, such as in the example shown in Figure 88, until metamorphosis is complete. Otherwise, they can remain in the larval tanks until settlement is complete. Since they are of similar size and behaviour to scallop pediveligers, similar densities can be set per unit area of the trays. Although adult clams bury themselves in the substrate in nature there is no need to provide substrate until the spat exceed 7 mm shell length. Settled spat can be removed from surfaces with a water jet.
Mussels also attach by means of byssus threads, but more strongly than scallops and clams and they retain their ability to form such attachments throughout their life. Because of their low per unit value compared with oysters, scallops and most of the commercial clams, hatchery culture is less common. Mussel spat are usually collected in nature although interest in hatchery production is now being shown on the West Coast of the USA and in New Zealand. Panels or coils of the same materials used to catch wild spat can be used in the hatchery, including rope, netting and panels of plastic mesh. The type of system with deeper tanks shown in Figure 87 is equally as appropriate for the settlement of mussel larvae as it is for scallops.
How spat are grown once they have settled is dealt with in the next section.