6 APPENDIX: THE MAIN GELATINOUS PLANKTERS OF THE MEDITERRANEAN AND THE BLACK SEA
For each species1, the following information is given:
• Geographic status (in respect the Mediterranean and Black Sea region): Indigenous, non indigenous, cryptogenic.
• Ways of introduction: (If indigenous, not applicable).
• Life cycle
• Presence patterns: regular - occasional - seasonal.
• Behaviour: e.g. vertical migrations.
• Ecophysiology
• Genetic characterization
• Association with other species
• Diet: food, nutrients.
• Predators
• Documented blooms (one, several, many)
• Geographic distribution in the Mediterranean and the Black Sea • Documented impacts
• Possible impacts
• Gaps in knowledge
• Management measures
1 All pictures provided are by A. Gennari.
Aurelia aurita (Linnaeus, 1758)

Geographic status: indigenous.
Ways of introduction: not applicable.
Life cycle: typical scyphozoan life history, with benthic scyphopolyps that asexually strobilate ephyrae that grow into sexual medusae, the females of which brood larvae that settle into the shallow coastal benthos within a few days of being released (Dawson and Jacob, 2001). Large developmental plasticity (e.g. podocysts, pseudoplanulae, reverse development) (Piraino et al., 2004).
Presence patterns: the main period of strobilation, resulting in release of ephyrae, starts in the late winter/early spring. The ephyrae released develop into medusae by early spring, which endure till summer or early autumn. Prolonged or even semi-continuous periods of strobilation have been reported in some areas, resulting in the presence of ephyrae in the water column for much of the year (Lucas, 2001). Occasional medusae in midwinter (Galil et al., 1990).
Behaviour: very common in the mixed layer down to the subthermocline in the Black Sea. Small individuals are mostly found above the thermocline, while larger individuals (up to 40 cm umbrella diameter) below (Bat et al., 2009). Exhibits a consistent pattern of diel vertical migration (Malej et al., 2007).
Ecophysiology: poorly known.
Genetic characterization: molecular studies revealed cryptic species (Dawson and Jacob 2001). Association with other species: unknown.
Diet: small copepods, copepodites, larvae of Gastropoda, Bivalvia, Cirripedia, nauplii, Appendicularia, fish eggs and larvae (Malej et al., 2007).
Predators: probably fish, not well documented.
Documented blooms (one, several, many): many, both in the Mediterranean and the Black Sea. Geographic distribution in the Mediterranean and Black Sea: common inhabitant of nearshore waters circumglobally between about 50 °N and 55 °S (Turk et al., 2008; Dawson and Jacob, 2001). Common in the Adriatic Sea, also in closed basins (Mliet Island, Lake of Varano). Occasionally abundant in the Black Sea and in the western and central Mediterranean.
Documented impacts: Aggregations may clog cooling water intakes of coastal power plants and block fishing nets (Dong et al., 2010).
Possible impacts: impoverishment of plankton communities.
Gaps in knowledge: distribution of polyps (Di Camillo et al., 2010).
Management measures: reduce polyp growth.
Cotylorhiza tuberculata (Macri, 1778)

Geographic status: indigenous.
Ways of introduction: not applicable.
Life cycle: Occurrence, growth, maturation, and aging of medusae indicate an annual life cycle. Ephyrae are released during strobilation peaks in spring and summer; exceptionally high growth rates lead to medusa diameters of up to 40 cm after six months. Due to symbiotic zooxanthellae, the medusae are potentially autotrophic. The gonochoristic medusae mature during summer; a sexual dimorphism is evident by brood-carrying filaments in females. The life span of the medusae is about half a year, while scyphistomae are potentially perennial. The observed annual metagenetic cycle is a life history adaptation to a highly seasonal environment (Kikinger, 2008).
Presence patterns: June to September (Perez-Ruzafa et al., 2002), occasionally in winter (Galil et al., 1990).
Behaviour: poorly known.
Ecophysiology: poorly known.
Genetic characterization: poorly known.
Association with other species: juvenile fish are often associated with it, and it might enhance recruitment success in some species.
Diet: microphagous and photosynthetic due to the presence of symbiotic microalgae. Predators: Caretta caretta (Revelles et al., 2007).
Documented blooms: formed blooms in the past, although they were not documented very frequently (Kogovsek et al., 2010).
Geographic distribution in the Mediterranean and Black Sea: throughout the Mediterranean Sea (Galil et al., 1990).
Documented impacts: none.
Possible impacts: it might have a positive effect on fish recruitment by providing shelter to juveniles. Gaps in knowledge: impact on food chains.
Management measures: limit substrates conducive for polyp settlement, such as dead mollusc shells.
Mnemiopsis leidyi (Agassiz, 1865)
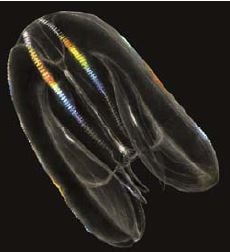
Geographic status: non indigenous; originating from the western Atlantic (Purcell et al., 2001), in coastal waters over a wide latitudinal range (40°N–46°S), it invaded the Black Sea in the 1980s, followed by subsequent invasions of the other large water bodies in the Mediterranean basin (Shiganova et al., 2001, Galil et al., 2009).
Ways of introduction: ballast water (Shiganova, 1998).
Life cycle: size changes from 0.5 mm to more than 50 mm in length and development from the cydippid larval stage to adult lobate morphology (Rapoza et al., 2005).
Presence patterns: population sizes in temperate locations small during cold winter temperatures, and increase with reproduction in the spring (Kremer, 1994).
Behaviour: usually at a depth shallower than 20 m during all months. The ctenophore was found in the deepest layer, at 50–100 m, only in summer months (Roohi et al., 2010).
Ecophysiology: metabolism and growth of M. leidyi are clearly influenced by temperature (Kremer, 1977). Mnemiopsis is found in an extremely wide range of environmental conditions (winter low and summer high temperatures of 2°C and 32°C, respectively, and salinities of 2–38 per thousand) (Purcell et al., 2001).
Genetic characterization: two of the sequenced ctenophores (SAL-1 and HAF-1) contained an ITS composite genotype that was previously found in invasive M. leidyi from the Black Sea (south western Black Sea and Gelendzhik Bay, Russia) and the Sea of Azov (various locations), as well as in native ctenophores from the United States, possibly indicating common recent ancestry (Fuentes et al., 2009).
Association with other species: unknown.
Diet: feeds on a variety of prey (Larson, 1988); Cladocera, copepods, bivalve larvae, crab larvae, diatoms, dinoflagellates, fish eggs and fish larvae (Purcell et al., 2001).
Predators: Beroe ovata, Chrysaora quinquecirrha, Cyanea capillata, Peprilus alepidotus and butterfish Pronotus triacanthus (Fuentes et al., 2010).
Documented blooms: blooms of the invasive ctenophore, Mnemiopsis leidyi, occurred in 2009 along the Mediterranean Sea coasts of Spain and Israel (Galil et al., 2009; Fuentes et al., 2010). In the framework of the CIESM Jellywatch campaign in the summer of 2009, M. leidyi was recorded from the Ligurian, Tyrrhenian, and Ionian Seas, including swarming episodes that, together with those reported from Spain in the same period, suggest a great success of the species in the estern Mediterranean (Boero et al., 2009).
Geographic distribution in the Mediterranean and Black Sea: present since more than two decades in the Black Sea, became abundant in the whole Mediterranean since 2009.
Documented impacts: invasion of regions outside its historical distributions have resulted in dramatic planktonic community alterations and destruction of fisheries in regions such as the Black Sea (Shiganova et al., 2003). Interfered with operation of desalination plants in Israel (Galil et al., 2009). Possible impacts: unknown.
Gaps in knowledge: establish how many colonization events did occur, was there an adaptation to Mediterranean conditions?
Management measures: discharge ballast waters in the mid-Atlantic and fill ballast tanks in regions where putative aliens are less frequent.
.
Pelagia noctiluca (Forskal, 1775)

Geographic status: indigenous.
Ways of introduction: not applicable.
Life cycle: holoplanktonic.
Presence patterns: regular, mostly in summer, first sightings in February, present until October. Possibly, it spends the winter in deep water, or as resting stages. Occasionally winter swarms (Galil et al., 1990).
Behaviour: forms enormous swarms, carried by currents, known to move vertically in the water column, especially in winter.
Ecophysiology: poorly known.
Genetic characterization: mixed populations throughout the Mediterranean (Stopar et al., 2010). Association with other species: unknown.
Diet: zooplankton, including ichtyoplankton.
Predators: several fish species (undocumented quantitatively).
Documented blooms: many.
Geographic distribution in the Mediterranean and Black Sea: abundant especially in the western Mediterranean, occasionally in the eastern basin.
Documented impacts: human health, tourism, fisheries, aquaculture, power plants. Possible impacts: not applicable (all the negative impacts of jellyfish are directly applicable to this species, so no putative ones remain).
Gaps in knowledge: where are they when they are not present? Are there areas from where the blooms spread? Are there resting stages?
Management measures: reduce overfishing, especially of medusivorous species. Instruct first aid stations on beaches how to alleviate stings. Increase public awareness.
Phyllorhiza punctata (von Lendenfeld, 1884)

Geographic status: non indigenous.
Ways of introduction: unknown. The records from the Levantine Basin suggest entry from the Red Sea through the Suez Canal, whether by drift or transported by vessels (ephyrae with ballast water, scyphistomae attached to hulls) (Abed-Navandi and Kikinger, 2007; Galil et al., 2009). Life cycle: poorly known for the Mediterranean (see Garcia, 1990; Ripingale and Kelly, 1995). Presence patterns: found off the Israeli coast in January, July and October (Galil et al., 2009). In Brazil, the presence in late winter and spring of all size classes suggested a prior period of continuous ephyrae release synchronized to seasonal high water temperatures and extended photoperiod (Haddad and Nogueira, 2006).
Behaviour: unknown.
Ecophysiology: poorly known.
Genetic characterization: Bayha and Graham (2009) characterized the polyps. Association with other species: algal endosymbionts (zooxanthellae) (Garcia, 1990; Galil et al., 2009) and thus autotrophy may be important for this species.
Diet: zooplankton.
Predators: unknown.
Documented blooms: several (e.g. Gulf of Mexico), but not in the Mediterranean Sea. Geographic distribution in the Mediterranean and Black Sea: both in the eastern and the western basins, so far only occasional.
Documented impacts: none in the Mediterranean Sea. In the Gulf of Mexico it clogged fishing nets.
Possible impacts: may harm fisheries by predating on fish eggs and larvae and their prey, zooplankton (Boero et al., 2009).
Gaps in knowledge: very little is known about this species in the Mediterranean Sea. Management measures: common in marine aquaria displays. Control aquarium trade.
Physalia physalis (Linnaeus, 1758)

Geographic status: non indigenous, native of tropical and subtropical areas (Wilson, 1947). Ways of introduction: enters the Mediterranean occasionally, through the Strait of Gibraltar (suggested by distribution patterns).
Life cycle: an "individual" is actually a colony of unisexual organisms. Every individual has specific gonozooids (sex organs or reproductive parts of the animals, either male or female). Each gonozooid is comprised of gonophores, which are little more than sacs containing either ovaries or testes. Physalia are dioecious. Their larvae probably develop very rapidly to small floating forms. Fertilization is assumed to occur in the open water, because gametes from the gonozooids are shed into the water. This may happen as gonozooids themselves are broken off and released from the colony. The release of gonozooids may be a chemical response occurring when groups of individuals are present in one locality. Critical density is probably required for successful fertilization. Fertilization may take place close to the surface. Most reproduction takes place in the fall, producing the great abundance of young seen during the winter and spring.
Presence patterns: occasional in the Mediterranean Sea, more abundant in recent years. Behaviour: transported by wind, it floats on the surface.
Ecophysiology: powerful venom, occasionally lethal.
Genetic characterization: poorly known.
Association with other species: Lepas fascicularis and L. pectinata, Caretta caretta, Nomeus gronovii (Wilson, 1947).
Diet: larval fish comprised 70 to 90 percent of the prey types found in stomach contents of Physalja physalis. It feeds also on chaetognaths and small squids (Purcell, 1984).
Predators: Glaucus atlanticus, Janthina janthina, Caretta caretta.
Documented blooms: several, especially in the Atlantic, but also in the Mediterranean Sea. Geographic distribution in the Mediterranean and Black Sea: mostly in the western basin, but may pass Sicily Channel and reach Malta.
Documented impacts: lethal (Stein et al., 1989), one case reported in the Mediterranean (Sardinia, summer 2010).
Possible impacts: potential impact on commercial fishing in the area. A popular food choice for the Man o’ War is larval fish: if too many fish are consumed in their larval stage, there won't be many adult fish for humans to harvest.
Gaps in knowledge: dependence of Mediterranean populations on Atlantic propagules, impact on food chains.
Management measures: close bathing facilities when the species is present. Collection and disposal on land.
Rhizostoma pulmo (Macri, 1778)
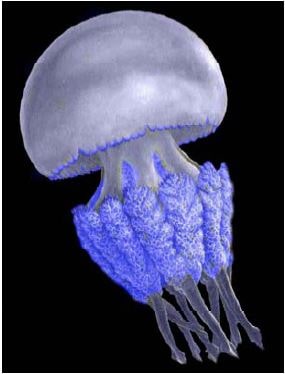
Geographic status: indigenous.
Ways of introduction: not applicable.
Life cycle: Fertilization external, planulae appear after 2 days, settle polyps reproduced asexually mainly by podocysts. Strobilation is induced by temperature cue. During transformation from newly released ephyra to young medusa, velar lappets appear and increase in number (Paspaleff, 1938). Reverse transformation of ephyrae into scyphopolyps has been observed (Paspaleff, 1938).
Presence patterns: seasonal, occurring usually in late spring when the temperature increase to 25.5°C (Galil et al., 1990; Mariottini and Pane, 2010).
Behaviour: poorly known.
Ecophysiology: swarms seemed to correlate with high temperature and nutritional factors connected to the abundance of zooplankton, which is the food for this microphagous jellyfish (Mariottini and Pane, 2010).
Genetic characterization: unknown.
Association with other species: the crab Liocarcinus vernalis is often transported by this jellyfish. Diet: diatoms (Lilley et al., 2009).
Predators: unknown, probably many fish species.
Documented blooms: annual blooms (Kogovsek et al., 2010). During the years of the bloom in the Mediterranean Sea, Rhizostoma pulmo occurred in large numbers in the northern Adriatic Sea, in open sea and along the coastline, as well as in the southern Adriatic Sea and the northern Ionian Sea, mainly in winter. Rhizostoma pulmo was indicated to be the largest and most abundant jellyfish in Lebanese coastal waters, occurring usually in late spring when the temperature increase up to 25.5°C, staying in Lebanese waters up until mid-August and disappearing later on (Mariottini and Pane, 2010).
Geographic distribution in the Mediterranean and Black Sea: mainly Adriatic Sea, Ionian Sea, Ligurian Sea, eastern Mediterranean, Tunisian waters, western Mediterranean and Black Sea (Mariottini and Pane, 2010). During the last decade, in some eastern Mediterranean waters Rhizostoma pulmo has been replaced by Rhopilema nomadica (Herut and Galil, 2000).
Documented impacts: many economic problems and also health implications (Mariottini and Pane, 2010). After a contact cutaneous pain, erythematous with subsequent small vesicles (Kokelj and Plozzer, 2002). The stings are much milder than those of Pelagia noctiluca.
Possible impacts: unknown.
Gaps in knowledge: triggering of blooms.
Management measures: reduce eutrophication, utilization as a food.
Rhopilema nomadica (Galil, 1990)

Geographic status: East African species. It was first recorded along the coastlines of Israel in 1977 (Galil et al., 1990). At present is found along the Levantine Basin with a single record off the Peloponnesus, Greece (Galil et al., 1990; Siokou-Frangou et al., 2006).
Ways of introduction: invasion through the Suez Canal.
Life cycle: life cycle from planula to ephyra to young medusa described. Strobilation considered dependent on temperature, with rapid strobilation between 18–20°C and declining at 24–26°C. The rise of water temperature supports the strobilation in spring, while inhibited in winter and in summer (Lotan et al., 1992).
Presence patterns: huge swarms are formed each summer since the mid-1980s along the SE Levantine coast (Galil et al., 1990; 2010).
Behaviour: poorly known.
Ecophysiology: laboratory studies support the possibility that synchronization and annual occurrence are controlled by seasonal changes in water temperature regimes, leading to rapid strobilation and release of ephyrae during springtime. The sensitivity of the polyps to low temperatures might explain why its dispersal is limited to the eastern Mediterranean (Lotan et al., 1994), but this sole reason is probably too simplistic.
Genetic characterization: unknown.
Association with other species: juveniles of Alepes djedaba, a carangid fish that entered through the Suez Canal, are commonly found in association with R. nomadica, taking shelter under its umbrella and among the filamentous mouth arms (Galil et al., 1990).
Diet: unknown.
Predators: unknown.
Documented blooms: many, in the Levantine Basin.
Geographic distribution in the Mediterranean and Black Sea: south-eastern Mediterranean. Documented impacts: the swarms adversely affect tourism, fisheries and coastal installations. The summer swarming results each year in envenomation victims suffering burning sensation, eurythema, papulovesicular and urticaria-like eruptions that may last weeks and even months after the event. Coastal trawling and purse-seine fishing are disrupted for the duration of the swarming due to net clogging and inability to sort yield. Jellyfish-blocked water intake pipes pose a threat to desalination plants, cooling systems of port-bound vessels and coastal power plants (Galil et al., 1990; Galil, 2007; Mariottini and Pane, 2010).
Possible impacts: impoverishment of plankton communities.
Gaps in knowledge: distribution of polyps triggering of blooms, competing species and predators, etc. Management measures: reduce suitable substrates for polyps, reduce overfishing.
Velella velella (Linnaeus, 1758)
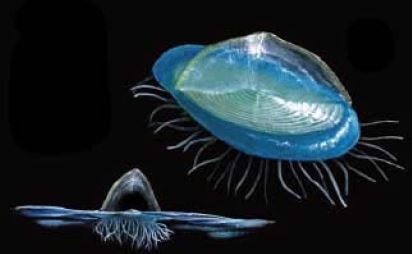
Geographic status: indigenous, circumglobal in warm and temperate waters. Ways of introduction: not applicable.
Life cycle: the floating stage is a polyp colony. It produces medusae that reproduce sexually (Larson, 1980), the larvae sink in deep water, and then migrate towards the surface while the colony is formed, going through several growth stages (Voltereck, 1904).
Presence patterns: present in spring, early summer.
Behaviour: wind-transported.
Ecophysiology: poorly known.
Genetic characterization: poorly known.
Association with other species: Scrippsiella velellae (Peridiniales) (Banaszak et al., 2006). Diet: iponeuston, including fish eggs and larvae.
Predators: Caretta caretta (Parker et al., 2005); Puffinus carneipes (Gould et al., 1997); Ianthina janthina (Bayer, 1963).
Documented blooms: many, also in the Mediterranean Sea.
Geographic distribution in the Mediterranean and Black Sea: eastern and western Mediterranean (Bouillon et al., 2004).
Documented impacts: stranded swarms form masses of putrescent material.
Possible impacts: might impair the recruitment of some fish species, if the blooms match the period of presence of fish eggs and larvae.
Gaps in knowledge: the studies on the life cycle are very old. Little has been done in recent years. Management measures: The stranded colonies enrich the sands of beaches (Kemp, 1986). This species is affected by oil pollution.