5.2 Production artificial reefs
5.2.1.Objectives
The overall objective of production artificial reefs is to increase the productivity of the aquatic environment and promote a sustainable utilization of resources.
When opportunely designed, artificial reefs may increase the biomass and hence the availability for human consumption of a variety of aquatic organisms (algae, molluscs, sea? urchins, fish) by enhancing their survival, growth and reproduction providing them with suitable habitats and additional food.
This type of artificial reef can also be used to manage the life stages of targeted species favouring aggregation of juveniles in certain areas and gathering the adults at suitable fishing grounds.
The specific applications of production artificial reefs include:
• recovery of depleted stocks by increasing juveniles survival providing them with shelter and additional food;
• enhancement of local fisheries by aggregating and establishing permanent populations of fish at suitable fishing grounds;
• shifting the fishing effort from an overexploited resource to other resources; e.g. if the soft?bottom associated species in an area are overexploited, artificial reefs can serve to shift a part of the fishing effort to pelagic or reef?dwelling species;
• compensation for a reduction of fishing effort: when there is a need to reduce fishing effort of trawling in an area, production artificial reefs can be used in negotiation to create new fishing grounds allowing fishers to shift towards more selective fishing activities;
• development of extensive algae and molluscs aquaculture, providing suitable substrates for settlement.
5.2.2.Design and materials
The modules that are generally used for production artificial reefs should be alveolar, of various shapes, and their surface area and niches (of various shapes and sizes) should be appropriate for the establishment of settling organisms. Unlike protection reef units, production units have usually more volume in relation to their weight, hence creating the three?dimensional complexity and developing surfaces which can be colonised by sessile organisms (Fig. 5). Rough surface texture enhances benthic settlement as it provides refuge and supports a greater diversity (Harlin and Lindbergh, 1977; Hixon and Brostoff, 1985; Beserra Azevedo et al., 2006). Consequently, this also affects the fish assemblage attracting fish grazing.
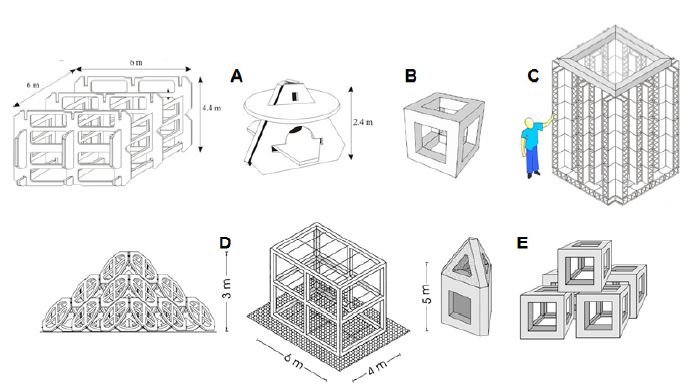
Figure 5. Examples of production artificial reef modules: A) France; B) Tunisia; C) Spain; D) Italy; E) Turkey (modified from E. Charbonnel, N. Haddad, J.J. Goutayer Garcia, CNR?ISMAR Ancona and A. Lok).
Food availability in the production artificial reef, as well as the composition, diversity and abundance of reef fish are strongly influenced by the occurrence of adequate refuges and by the shape of the structures. Habitat quality affects habitat selection by fish and, consequently, influences demography and population dynamics of the reef fish assemblage. Hence, to host a permanent community, an artificial reef must provide adequate habitats to juveniles and adults. On the basis of the fractal crevices theory in structurally complex natural or artificial environments, large crevices are much rarer than the smaller ones. Therefore, the artificial reefs can host a greater number of small and medium?sized organisms than large ones which tend to migrate outside. Therefore, the placement of large? holed reef units (especially in MPAs) could avoid depletion of broodstock by fishing and enhance the reproductive capacity of reef fish (Caddy, 2011).
Other factors that should be taken into account in planning artificial reef structures are:
• Regardless of their size and life stage, fish generally prefer cavities where there is light and with many openings that enable them escaping from predators;
• the size, number and orientation of cavities should match with the behavioural features of the target species, such as whether they are territorial or gregarious; • the overall design of artificial reef structures should assure adequate water circulation; • if demersal species are targeted, the structures design should consider providing vertical cover such as an “overhang” or shading from above as demersal fish will more readily utilize structures which provide protection from predators foraging from above.
With regard to the shape of the reef units/reef sets, it is well known that the affinity of several aquatic organisms towards the artificial substrates vary widely depending on the species and the life stage. Because of this, when constructing a reef for fisheries enhancement, it is important to deeply know the ecology of the different species so to identify those that are more appropriate as targets for artificial reef deployment and will have a higher probability of being manageable through manipulations involving artificial reefs.
Fish species have been classified according to their affinity to artificial reefs (Nakamura, 1985; Grove et al., 1991; Bortone, 2011; Fig. 6):
• Type A: benthic, reef?dweller organisms (fish, crustaceans, cephalopods) that prefer to live at strict contact with the substrates or inside holes (e.g. gobids, blennids, scorpenids, octopus and lobsters);
• Type B: nekto?benthic, reef?dweller fish that swim around the structures but are linked to them by the occurrence of shelter and/or prey availability (e.g. sparids, sciaenids, seabass and labrids);
• Type C: pelagic fish swimming in the middle and surface layers of the water column; they usually maintain a certain distance from the artificial structures but are likely to be linked to them by vision and sounds (e.g. mugilids, amberjacks and dolphin fish);
• Type D: species that are found on, in, or over the substrate next to the reef. These species have similar needs to C?type species but they live on or above the substrate surrounding the reef (e.g. bothids).
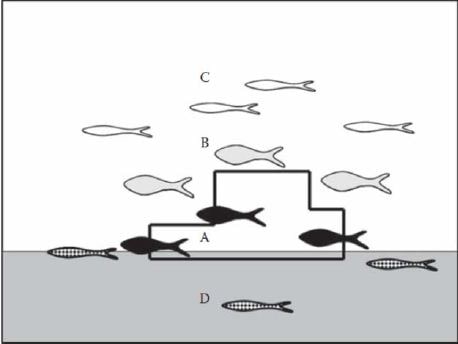
Figure 6. Classification of fish according to their position relative to the artificial reef (from Bortone, 2011).
In Figure 7, the different fish categories are displayed along two axes (attraction and production) according to their level of affinity towards hard substrates. C and D?type species are characterized by high attraction and low production relationships with artificial substrates, hence they are clearly not suitable to be managed with an artificial reef in terms of increasing production as these species are chiefly attracted to the reef. A?type species, which have a strong production relationship (e.g. spiny lobster or octopus) might gain a significant advantage from artificial reef deployment, while B?type fish will get benefit from artificial reefs depending on their life history strategies.
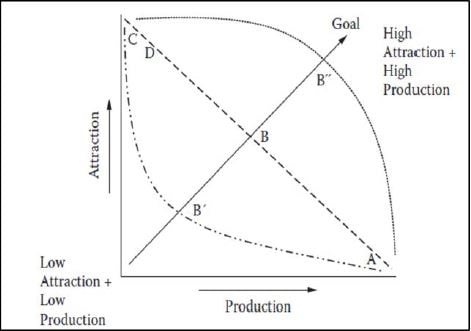
Figure 7. Relationship of A, B, C and D?type artificial reef species relative to attraction and production features of artificial reefs. B? and B? indicate the position of B?type species with different life history strategies (from Bortone, 2011).
To attract A?type organisms, the artificial reef structures do not need to extend vertically into the water column but should be provided with internal spaces matching with the size of the target species, while for B?type fish species the holes should be larger and the artificial reef structures must reach at least a height of 2 m. To aggregate C?type species, the artificial reef should extend vertically into the water column and its structures should have wide open spaces to favour the water flow. Simple units can be also used for particular species, e.g. clay jars for octopus. Consequently, the complexity and diversity of the fish assemblage associated to an artificial reef strictly depends on the complexity of the reef.
5.2.3.Siting
The displacement of reef structures within an artificial reef may affect its influence on fish. Greater distance between the reef units/reef sets may increase the total volume of the artificial reef but account should be taken of the fact that the effects on fish may be reduced if the units are placed too far from each other. The reef groups may act as isolated reefs (in space) if spaced too far apart. Correct spacing of reef units (or modules), reef sets and reef groups will ensure that the reef complex operates as one reef with fish readily transiting between refuge/predation points, which can greatly improve the “productive” value of the overall reef complex itself. The area of influence (or halo) of each unit, set and group will vary depending on target species and module design.
In general, the criterion to be applied in positioning artificial reef structures within a reef group is that the areas of influence of individual reef units and/or reef sets should overlap with each other (Grove et al., 1991). The reef groups do not need to interact with each other when included within a reef complex (Fig. 8).
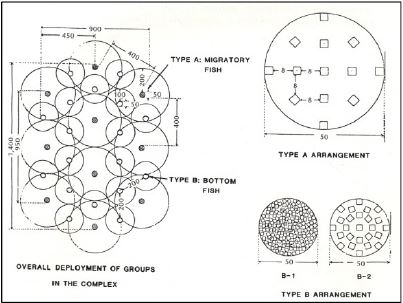
Figure 8. Spatial arrangement of reef units/reef sets in a reef complex (from Grove and Sonu, 1985).
Production artificial reefs should be placed in areas where stocks of target species already exist. Moreover, the reefs should match with the ecological requirement of those species. Usually in the Mediterranean Sea, this type of artificial reefs is placed in coastal waters up to 30 m depth, but the range depth can be appreciably greater in other seas (e.g. off Japan) where high relief artificial reefs are placed up to 80 m depth.
In the case of production artificial reefs aimed at enhancing and managing local fisheries, shifting the fishing effort helps compensate for the loss of fishing grounds due to other human activities. The choice should be towards B?type species which are attracted to reefs to a limited degree but also gain some production benefit from reef platform. With respect to the above?mentioned criteria, to assure stability and ecological effects, the artificial reefs should be placed as close as possible to the fishing harbours, hence allowing equitable and economic access by reducing travel and search times, saving on fuel and increasing fishers’ safety.
Artificial reefs may also help localize and manage the entire life cycle of some target fish. In this case, different reefs – each matching with the ecological requirements of a certain life stage of the species – should be deployed along the movement routes to gather the specimens in localized areas.
5.2.4.Practical applications
France
Among the 94 000 m3 of artificial reefs existing in France, one third concerns the Marseille reef complex, which is the largest artificial reef deployed in the Mediterranean Sea with 27 300 m3 covering 220 ha and conceived by marine biologists (Charbonnel et al., 2011). The reef deployment relied on the creation of horizontal and vertical discontinuities in heights, sizes and volumes thanks to a great variety of reef types and shapes, as well as on diverse arrangements and horizontal spacing of reef units/reef sets. Six types of modules of different shapes, sizes, volumes and materials were specially designed for this project (Fig. 9). To optimize the reef habitat diversity, the complexity of these modules was enhanced adding several types of small filling materials (bags containing oyster shells, breeze blocks, octopus pots used for fisheries) and floating immersed ropes. Piles of quarry blocks of variable sizes were also used to reconstitute natural rocky boulders.
The different modules were grouped in six triangular shaped reef groups (300 m). These groups were linked together by series of reef structures (functional connections) functioning as biological corridors and stepping stones for fish and propagules. The locations of peripheral natural habitats (Posidonia meadows and rocks) were taken into account in the arrangement of the reef groups to favour a rapid colonization of the artificial reef (Fig. 9).
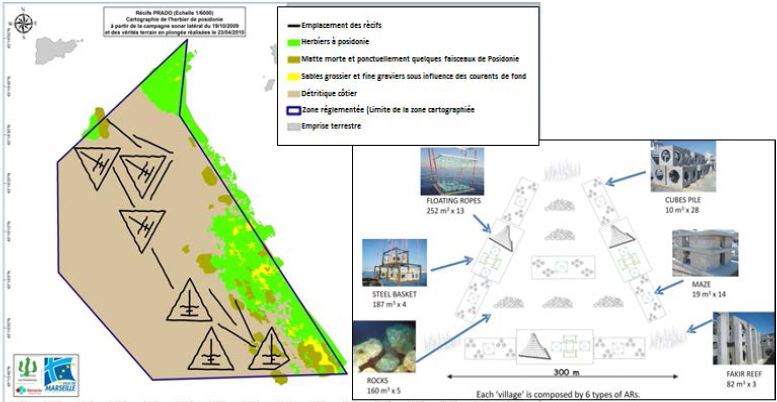
Figure 9. Marseille Prado artificial reef, France, the largest reef in the Mediterranean. Deployed in 2007–2008, it is composed of six “villages” linked by eight connections (above in green: lower limit of Posidonia meadow). Each village has a triangular shape and is made with six types of artificial reefs (below) (from Charbonnel et al., 2011).
Greece
Four multipurpose artificial reefs were constructed in 2000–2006 for the protection and management of marine resources. The reefs have a surface area of 8–10 km2 each and are made of different concrete modules. These are either mixed modules consisting of concrete cubic blocks with holes, deployed one by one on the seabed or assembled in pyramids, or production modules such as bulky cement?bricks on a concrete base and concrete pipes assembled in pyramids (Fig. 10).
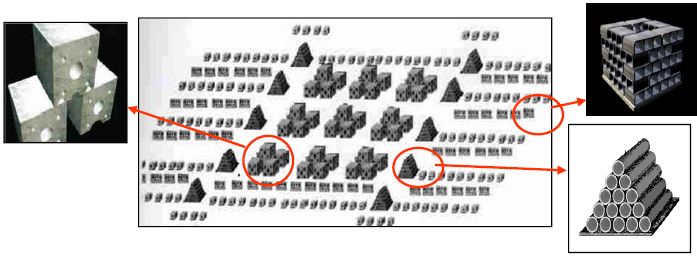
Figure 10. An artificial reef plan using four different types of modules in order to increase the reef complexity, Greece (modified from A. Kallianotis).
Turkey
Octopus species are habitat?dependent and of great economic interest. Despite a lack of specific information, data show that the individual weight of octopus has decreased over the last few years both globally and in Turkish seas. Furthermore, natural shelters of Octopus vulgaris along the Aegean coast of Turkey are often disturbed by spearfishers. For these reasons, a plan was created to deploy an artificial reef specifically designed for this species (octo?reefs). The goal was to provide octopus individuals with suitable habitats and to increase their population in the long term. Simple concrete modules with holes were placed on the sea bottom. The first results showed that octo?reefs were actually used by octopus (Fig. 11 and Plates 1 and 2). Hence, the next step will be to deploy this type of artificial reefs in a closed fishing area and in MPAs.
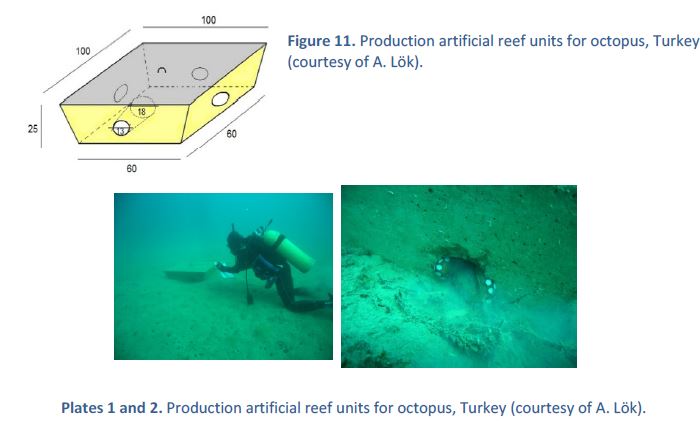
Figure 11. Production artificial reef units for octopus, Turkey
(courtesy of A. Lok).
Plates 1 and 2. Production artificial reef units for octopus, Turkey (courtesy of A. Lok).
Japan
Artificial reefs aimed at managing the life cycle of migratory fish were constructed in a bay of the Iki Islands (Sea of Japan), where schools of snapper (Sparidae) were observed to follow a migratory route coinciding with the propagation of waves inside the bay. The strategy adopted was to place a production artificial reef at the entrance of the bay, a spawning reef where the waves converged, and a nursery reef to improve the survival of juveniles (Fig. 12). This confined the life cycle of snapper into the bay, which considerably improved their survival, and allowed their catches to be managed by the local fishing communities (Nakamura, 1985).
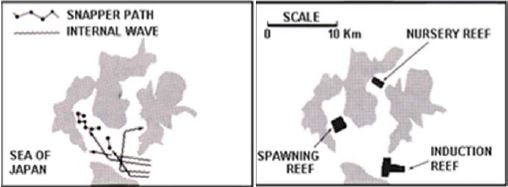
Figure 12. Deployment of artificial reefs aimed at managing the entire life cycle of snapper (from Nakamura, 1985).
Similar applications could be adopted in the Mediterranean and the Black Sea to manage the life cycle of some commercially important species, the juveniles of which, for example, prefer low depth and migrate towards offshore as they grow. A restocking experiment conducted with juveniles seabass (Dicentrarchus labrax; 15 cm TL) released in an artificial reef located at 11 m depth in the northern Adriatic Sea demonstrated that, just after release, the fish migrated inshore, close to estuarine areas. In the following months, during its growth, the fish migrated again to the artificial reef and the mussel cultures located between 10 and 13 m depth. In this case, the placement of suitable artificial reefs between the coast and the 13 m bathymetry could partially confine released seabass (Grati et al., 2011).