1.2 SCIENTIFIC BACKGROUND – NATURAL HABITAT AND REPRODUCTIVE CYCLE
1.2.1 Habitat
The scallop, Euvola (Pecten) ziczac (L.), also known as the sand scallop, zigzag scallop or Bermuda scallop, is a sub-tropical and tropical species (Figure 1.1). It is similar to other pectinids in that the right (lower) valve is very convex, whereas the left (upper) valve is usually flat but has been seen to slightly convex or concave in some cases. It has been fished recreationally and/or commercially in Brazil (Pezzuto and Borzone, 1997) and along the Caribbean coast of Venezuela and Columbia (Velez and Lodeiros, 1990), and has also been seen off Florida (USA) as a by-catch in the calico scallop fishery.
Its northernmost distribution is Bermuda (Lodeiros et al. 1989).
In Bermuda, the sand scallop inhabits protected inshore waters, lying on grassy, sandy bottoms, ranging in depth from 2–10 m (Sterrer, 1986). In its natural state, this scallop is recessed in the sand with the rim of its outer left valve showing. It

Figure 1.1: Photograph of a live E. ziczac, the sand scallop or zigzag scallop.
will swim when disturbed, but does not cover great distances. It has also been observed to bury completely 5–10 cm into the sand when faced with unfavourable conditions.
Maximum shell height recorded in Bermuda is 130 mm (Sterrer, 1986). Its life span is thought to approximate 5 years. Worldwide, population numbers of E. ziczac have been reported to be low, with a decline seen in the 1990’s. In Bermuda, it was known to occur in relatively large abundance during the 1940’s and 1950’s, and was recreationally fished until the early 1970’s. It since has been recorded here and there, with no real evidence of a self-sustaining population. To our knowledge, there is currently no existing commercial hatchery for this species.
The calico scallop, Argopecten gibbus (L.), is largely restricted to the sub-temperate and tropical waters of the western North Atlantic with major stocks distributed from Cape Hatteras, North Carolina (USA) to the Cape San Blas areas of the northeastern Gulf of Mexico (Waller, 1969). Calico scallops have also been collected from the Greater Antilles, Bermuda, and the western portions of the Gulf of Mexico (Waller, 1969). Commercially important stocks are located off North Carolina and northeastern Florida (USA), where it supports a small and transient fishery (Figure 1.2) The calico scallop has two convex valves, although the right is slightly more convex than the left (Sterrer, 1986). The upper valve is usually mottled with a combination of brown, red, purple, yellow and white. It is found lying on top of the seabed in sandy, rocky and grassy substrates, and has been recorded in Bermuda in several inshore waters, as well as on the more exposed North shore of the Island. It attains a maximum height of 70 mm in Bermuda (Sterrer, 1986). This species, like the sand scallop, was a commonly found bivalve in Bermuda and supported a recreational fishery at one time. Population numbers are at present very low in Bermuda (Sterrer, 1986). There is no commercial hatchery for this species at present.
A generalized diagram of a pectinid is shown below (Figure 1.3) outlining some
Figure 1.2: Map indicating the distribution of the calico scallop, A. gibbus.
shell characteristics and scallop organs. A photograph of a dissected calico scallop is also inserted to show the colouration of the gonad when ripe, the muscle and gills.
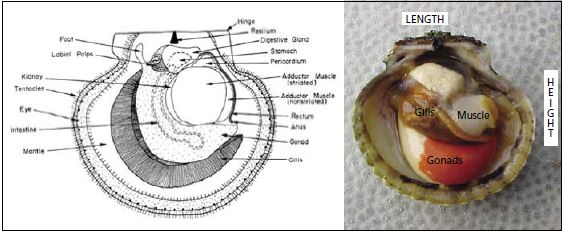
Figure 1.3: Generalized diagram of a pectinid (taken from Bourne, Hodgson and Whyte, 1989) alongside an open calico scallop specimen showing major organs.
1.2.2 Reproductive cycle
1.2.2.1 The sand scallop, Euvola (Pecten) ziczac
The reproductive cycle of E. ziczac in Bermuda has been determined based on histological analyses, gonadic index determinations and natural spatfall (Manuel, 2001). This species is a simultaneous hermaphrodite with whitish-yellow testis and orange ovaries when mature. According to Manuel (2001), gametogenesis is initiated in autumn and gonads mature during the colder winter months. High gonadic indices were determined by Manuel (2001) when seawater temperature was below 20 °C; this concurs with the observed peak spawning activity at the Bermuda hatchery between December and March. There was no major synchronized spawning recorded for this species in Bermuda waters. Animals tend to spawn sporadically subsequent to any small environmental changes, and very often were seen to release gametes partially rather than completely. This led to their classification by Manuel (2001) as “dribble spawners”.
Lodeiros and Himmelman (1994) found that E. ziczac in Venezuela attained full sexual maturity at a shell height of approximately 44 mm; this shell growth is obtained in Bermuda in the first 10–11 months, and allows for a first gametogenic cycle to concur with the first winter period (Sarkis, 1995). Latitudinal differences in the timing of the reproductive cycle are seen with this species as in the more southern region of the Venezuelan coast, where two major spawning events have been recorded for the sand scallop (Velez, Sotillo and Perez, 1987); the first occurring in April/May and the second during August/September, when sea temperatures range from 23–26 °C. Temperature and phytoplankton abundance are two environmental factors which have been cited as influencing gametogenesis and spawning in the sand scallop (Lodeiros and Himmelman, 2000; Velez, Alifa and Freites, 1993). Fecundity of E. ziczac when stimulated to spawn in Bermuda at the hatchery averaged 5 million eggs per female; similar results were recorded by Velez, Alifa and Freites (1993).
1.2.2.2 The calico scallop, Argopecten gibbus
The reproductive cycle of A. gibbus in Bermuda was determined using gonadic index and histological analyses. This species is also a simultaneous hermaphrodite with whitish testes and bright orange ovaries when mature (Figure 1.4). For ease of understanding, a description of the oocyte developmental stages is given in Appendix 1.
Timing for the initiation of gametogenesis and maturation of gametes is similar to the sand scallop, where gametogenesis is initiated in September and maturation reached during the winter months. The calico scallop in Bermuda exhibits a spawning period over the winter months, as for E. ziczac, associated with colder water temperatures and lower food availability. The difference between the two species is a more defined spawning period in

Figure 1.4: Calico scallop, A. gibbus, showing gonads with both mature ovaries (bright orange) and sperm (white).
A. gibbus, where release of gametes is complete and synchronous among the population, as observed in Bermuda. This is in agreement with Blake and Moyer (1991) who report, that a large percentage of any sub population of calico scallops in Florida (USA) waters normally spawns over a 1–3 week period. This definition is reflected in the specific peaks seen in gonad weight between February and April (Figure 1.5) and in the high percentage of ripe cells (70–90 percent) determined in histological sections of the ovary (Figure 1.6). Histological analyses suggest that the spawning period of A. gibbus in Bermuda ranges from December to possibly May. In the more southern waters of Florida (USA), Moyer and Blake (1986) reported two spawning periods for the calico scallop, the first in late spring (April to June), and the second in autumn. These latitudinal differences may be explained in part by environmental differences, namely those of food and temperature (Barber and Blake, 1983). The lack of a clear trend in muscle weight determined for A. gibbus in Bermuda suggests a direct dependence on food supply for gonadal development and maturation. Blake and Moyer (1991) found that the required threshold temperature for the same species in Florida (USA) was of 19–20 °C. Their
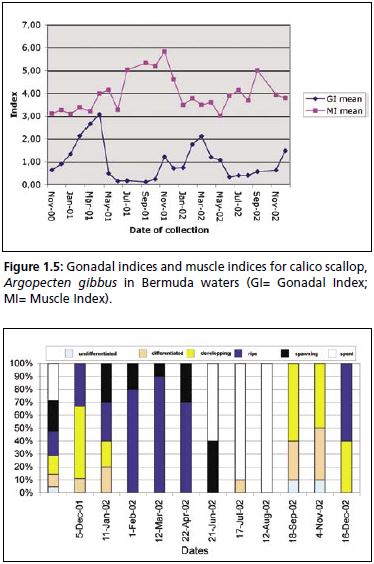
Figure 1.5: Gonadal indices and muscle indices for calico scallop, Argopecten gibbus in Bermuda waters (GI= Gonadal Index; MI= Muscle Index).
Figure 1.6: Reproductive patterns in cultured A. gibbus from Bermuda.
further conclusion that at temperatures above 22 °C maturation apparently stopped and spawning did not occur, concurs with results seen in the histological work and at the Bermuda hatchery, where a lack of gametogenic activity was seen during the warmer months, and spawning terminated in late spring (T=22±1 °C).
It has been noted by Blake and Moyer (1991) that an individual scallop normally reproduces for the first time at an approximate age of 6 or 12 months depending upon season of spawn. In Bermuda, 8 month old scallops have been observed to undergo gametogenesis and become reproductively mature within their first year of life (mean shell height of 47.4±2.2 mm). Each scallop is thought to spawn 2 or 3 times during its 18–24 months life span in Florida (USA) waters (Blake and Moyer, 1991). Although this has not been scientifically assessed in Bermuda, one cohort has been observed to spawn twice a year for a maximum period of three years. Fecundity of A. gibbus when stimulated to spawn in the Bermuda hatchery averages 6.26 million (n=5).
1.2.3 Life cycle
A generalized diagram of the life history of a scallop is provided below in Figure 1.7. Size shown for each stage is general, and differs among species. As seen in the above section, gamete release from ripe adults into the water, allows for external and controlled fertilization of the oocytes. Fertilized eggs or embryos are allowed to divide and develop into trochophore and early veliger larvae in larval tanks. The early larval stage is often referred to as D-larvae, as they take on a characteristic “D” shape, or straight hinge larvae, or Prodissoconch-I stage. Rearing of larvae is continued in these tanks, for development into umboned or Prodissoconch-II larvae. This hatchery stage lasts until larvae are mature and reach the pediveliger stage. At this time, larvae alternate from a swimming state to substrate-search behaviour by use of a newly developed foot. They may attach to various surfaces by secreting a byssus acting as temporary holdfast. Larvae are then ready to undergo metamorphosis, a critical time in their development. High mortalities may be seen at this time. Metamorphosed larvae settle, and are termed spat. They are reared in a nursery system until they are strong enough to be transferred to the field. Juveniles are reared in enclosures in the natural environment until adult size. Descriptions of techniques for larval, post-larval and juvenile culture are given in Chapters 3, 4 and 5.
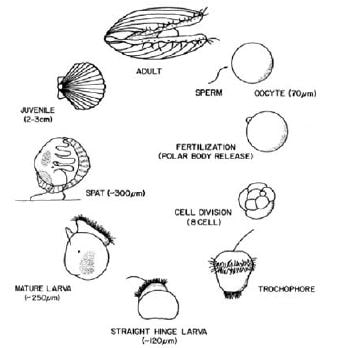
Figure 1.7: Generalized life history of a pectinid (taken from Bourne, Hodgson and Whyte, 1989).