3.2.1 Procedures for the management of stock cultures
It is necessary to sub-culture stock cultures at monthly intervals to maintain them in a vigorous and healthy state. Following removal of the cotton wool plug from a stock culture flask and flaming the neck of the flask with a Bunsen burner (or butane torch), an inoculum of 20 to 50 ml is decanted into another sterile flask containing autoclaved medium.
The plug is inserted after flaming of the neck of this new flask. Species name and the date are indelibly marked on the flask, which is then returned to the incubator. The original stock culture can be kept for a few weeks in the event that the new stock culture fails to grow. The stock culture transfer procedure is best performed in a cabinet that has been sterilized by ultra-violet light to further reduce the risk of contamination (see Figure 16). Details of the transfer procedure are given in the accompanying box.
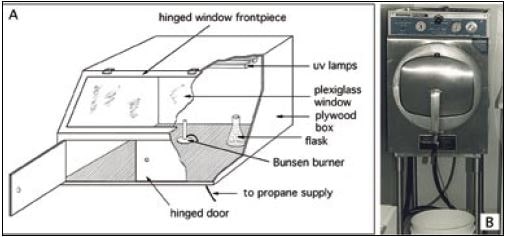
Figure 16: A – schematic diagram of a culture transfer chamber. B – an autoclave suitable for the
sterilization of small volumes of culture medium.
Table 2: The composition and preparation of Erdschreiber culture maintenance medium.
Constituents:
1. Seawater: Autoclave 2 l in a 3 l borosilicate glass flat-bottomed boiling flask with cotton wool plug at 1.06 kg cm-2 for 20 minutes. Stand for 2 days.
2. Soil extract: prepared as follows:
a) mix 1 kg soil from a woodland or pasture area untreated with artificial fertilizers, insecticides, etc. with 1 l of distilled freshwater;
b) autoclave at 1.06 kg cm-2 for 60 minutes;
c) decant off the supernatant liquid;
d) filter supernatant through Whatman No. 1 paper and then through a glass-fibre (GF/C) paper;
e) autoclave in 1 l aliquots in polypropylene bottles at 1.06 kg cm-2 for 20 minutes; f) store in deep freeze until required;
g) autoclave 100 ml in 500 ml borosilicate glass, flat-bottomed boiling flask with cotton wool plug at 1.06 kg cm-2 for 20 minutes.
3. Nitrate/phosphate stock solution: Dissolve 40g NaNO3 and 4 g Na2HPO4 in 200 ml distilled water. Autoclave in 500 ml flask at 1.06 kg cm-2 for 20 minutes.
4. Silicate stock solution: Dissolve 8 g Na2SiO3.5H20 in 200 ml distilled water. Autoclave in 500 ml flask at 1.06 kg cm-2 for 20 minutes.
Procedure:
Add 100 ml soil extract (2) to 2 l of sterilized seawater (1). With sterile pipette add 2 ml nitrate/ phosphate stock solution (3) and 2 ml silicate stock (4). Decant 250 ml into 8 empty autoclaved 500 ml flasks with cotton wool plugs. Use a Bunsen burner or butane torch to flame the necks of the flasks immediately before and after decanting/pipetting. The maintenance medium is now ready to use.
Procedure for transferring algal cultures from flask to flask
(a) Wipe all inner surfaces of inoculating booth with 85% ethanol.
(b) Place all flasks that will be required in the booth; i.e. all flasks to be transferred from (the transfer flask) and flasks containing sterilized media to be transferred into (new flasks).
(c) Close booth and switch on ultra-violet lamp. Leave for at least 20 minutes. (It is not safe to look directly at ultraviolet light, so a dark cover should be placed over the plexi-glass (transparent acrylic plastic) viewing plate when the light is on.)
(d) Switch off lamp. Ignite small burner.
(e) Remove foil caps from one transfer and one new flask. Flame the neck of each flask by slowly rotating the neck through the flame.
(f) Tilt the neck of the transfer flask toward the new flask. In one motion, remove both stoppers and pour an inoculum into the new flask. Transfer approximately 50 ml for diatom species and 100 ml for flagellates. Avoid touching the necks of the two flasks. Never touch the portion of the stopper that is inserted into the flask. Once the
inoculum is added, replace the stopper in the transfer flask. Slowly flame the neck of the new flask before replacing its stopper.
(g) Replace foil cap over the neck of the new flask. Using a waterproof marker pen, label the new flask with the algal species inoculated and the date of transfer.
(h) Repeat procedure for all flasks within the booth. Once completed, turn off burner and open booth.
(i) Remove all new flasks and place in the algal incubator or a well-lit area in the algae culture facility.
(j) The remaining inoculum in the transfer flasks can be used to inoculate larger cultures such as 4 l flasks or carboys.
(from: Bourne, Hodgson and Whyte, 1989)
Table 3: Guillard’s F/2 media used for culturing algae in bivalve hatcheries from Guillard (1975).
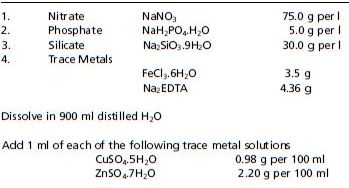

Table 4: HESAW media used for culturing algae in bivalve hatcheries. From Harrison et al. (1980).
