5.3.3 Seawater quality
It is generally the exception for a hatchery to operate at a constant rate of production throughout the year. Factors of a seasonal nature that cannot easily be controlled may bring about periods in the year when the performance of larvae in terms of growth rate and survival is significantly poorer than at others. In the absence of a technical explanation, such as filter failure or the corrosion of equipment – among other possibilities – or the use of poor quality algal cultures that may have become contaminated, or lapses in husbandry (human error), seawater quality may be responsible.
It has long been established that seawater varies seasonally in its ability to support the growth and survival of embryos and larvae. This may not happen globally, but adverse conditions do occur on both sides of the Atlantic Ocean, particularly when the sea begins to warm in spring and coincident with periods of intense phytoplankton blooming both in spring and in early autumn. The precise reasons for deterioration in seawater quality during these times are not completely understood and they may not recur annually. Some years are better than others in that respect.
By comparing the development of embryos or the growth of larvae week by week in normally treated hatchery seawater and in an artificial seawater control medium using standardized bioassay techniques it is possible to detect and quantify variations in seawater quality.
The methodology for bivalve embryo bioassays is detailed in Utting and Helm (1985). Adaptation can be made at the beaker or bucket scale to determine variability as it effects larval growth and survival. Artificial seawater can be prepared according to various recipes from analytical grade chemicals or be purchased as proprietary brands from laboratory suppliers and from fish hobbyist stores. It must always be prepared in the same way as the constant quality, control medium.
An example of seawater quality variability as it influences development of Pacific oyster embryos in the hatchery is given in Figure 74. The development of fertilized eggs to perfectly formed D-stage larvae is expressed as net treatment mortality (NTM), where
NTM = 1- {(Mean yield of D-larvae in 2 ml of normally treated SW) /(Mean yield of D-larvae in 2 ml of artificial SW)} -100
A net treatment mortality value of 0 indicates as great a number of fertilized eggs surviving to the D-stage in both media and an NTM of 100 denotes a total failure in development in the normally treated seawater. Negative values indicate that the hatchery water was superior to the artificial seawater.
In the early part of the year in northern temperate latitudes of the Atlantic Ocean, when sea temperatures are cool and day length short, seawater quality is relatively stable. As the coastal waters warm and day length increases towards and during spring and early summer, seawater quality becomes increasingly variable. NTM values begin to climb unpredictably and in some years there will be periods when it is difficult to produce D-larvae from apparently good quality eggs – eggs will develop normally in the artificial control medium but not in the hatchery seawater. The phenomenon applies equally to a wide range of bivalve species, not just to the Pacific oyster.
Unstable seawater quality is generally coincident with intense phytoplankton production in coastal waters during spring bloom conditions. There is no direct evidence that it is the metabolites or break down products of the phytoplankton that cause deterioration in water quality. Rather, it may be the bacteria associated with blooms or the metabolites, including the exotoxins, they produce.
A situation of this kind is illustrated in Figure 74 at a hatchery location where the dominant algal species towards the end of the spring bloom in coastal waters was the colonial flagellate, Phaeocystis pouchetti. Comparison of the NTM values for the different years shows that the quality of hatchery seawater was at its poorest when numbers of bacteria forming colonies on TCBS agar were at their highest. Bacteria that form colonies on this agar include species of the genus Vibrio. They include known opportunistic pathogens, such as V. anguillarum, which have frequently been reported as among the more dominant bacteria in hatchery systems at that time of year. They are often implicated in disease situations. V. anguillarum is known to produce potent exotoxins including a low molecular weight ciliostatic toxin that inhibits the beating of cilia of the velum in larvae and gills in juveniles.
Seawater quality for embryo development can be improved by various chemical pre treatments for 24-hour periods before use. This is in addition to the usual filtration and UV disinfection measures. Frequently effective treatments include the addition of 1 mg per l EDTA (ethylenediamine-tetraacetic acid) and 20 mg per l sodium metasilicate (Na2SiO3.9H2O) to tanks containing filtered seawater which are then vigorously aerated until required for use 24 hours later. The percentage of eggs that develop to the D-larva stage can often be significantly improved by such treatment. For example, in 28 trials with Pacific oyster embryos, yields of D-larvae from weekly spawnings
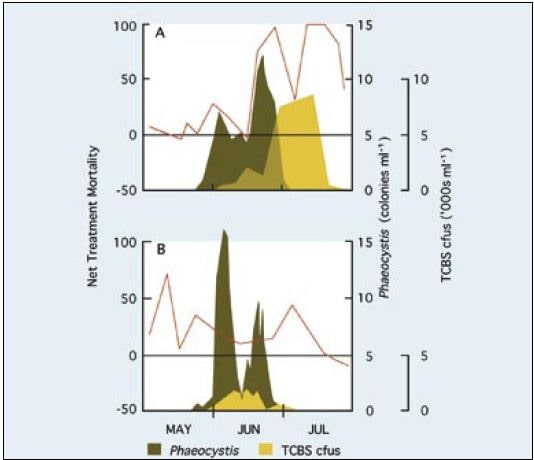
Figure 74: The relative survival (as net treatment mortality – red line) in bioassays comparing development to D-stage larvae of fertilized Pacific oyster eggs in artificial and in normally treated hatchery seawater during the period May to July, 1977 (A) and 1978 (B). The horizontal black line, equivalent to a net treatment mortality of zero, denotes equality in survival in both the tested seawater and the control medium. The blooming of the colonial flagellate, Phaeocystis pouchetti, (as colonies per ml) and the number of bacteria colonies (cfus – colony forming units – as thousands per ml) growing on TCBS agar in samples taken from the adjacent coastal waters are superimposed. Adapted from Utting and Helm (1985) including previously unpublished data.
throughout the hatchery season (March to September) were improved from a mean of 36.6% to 52.9%. The improvement through use of chemical pre-treatment compares with a mean yield of 54.6% in the artificial control medium.
The growth rate of larvae from the D-stage is also influenced by variability in seawater quality in much the same way and for the same reasons as is embryo development. Again, effects on growth have been evident in all species of bivalves tested. Comparative growth of Pacific oyster larvae over a 6-day period from the D-stage when cultured at the beaker scale at 25oC in normal hatchery water and in an artificial seawater preparation (Lyman and Fleming formula, from Sverdrup et al. (1942)) is shown in Figure 75. Differences in growth rate are expressed as Growth Index (GI), where:
GI = 6-d growth increment (µm) in hatchery seawater / 6-d growth increment (µm) in the artificial seawater control
Growth indices >1.0 are indicative of periods when growth was superior in hatchery water; a GI of 1.0 shows parity in performance between the two media and GIs of <1.0 denote times when growth rate was inferior in hatchery water compared with the artificial medium.
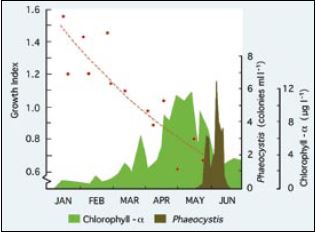
Figure 75: The comparative growth of Pacific oyster larvae over a 6-day period at 25oC in normal hatch ery seawater and artificial seawater calculated as a growth index (see text).
Chlorophyll–? and the numbers of Phaeocystis pouchetti colonies at the hatchery intake are shown as indicators of phytoplankton produc tion in the coastal waters adjacent to the hatchery. (M.M. Helm, unpublished)
Results shown in Figure 75 imply a progressive deterioration in seawater quality from the beginning of the hatchery season in January until trials ended in late May when – for a period of approximately 6 weeks – larvae failed to survive the 6-day experimental period in either medium. Until the end of April, batches of larvae reared for spat production developed normally to settlement in hatchery seawater and provided good yields of spat. Large-scale culture was problematic beyond that time with poorer survival and eventual failure of larvae to reach settlement. The same trend is apparent for European flat oyster, Ostrea edulis, larvae where deteriorating performance both in terms of growth (Figure 76) and survival often culminated in disease and the total mortality of broods of larvae in culture in May and June. Again, seawater quality was more variable in some years than in others.
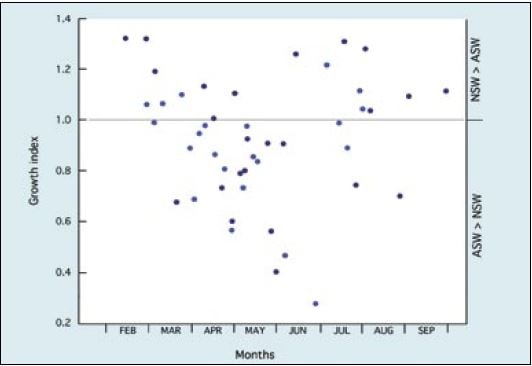
Figure 76: Growth indices of samples of broods of European flat oyster, Ostrea edulis, larvae grown at the beaker scale in hatchery and artificial seawater for a 4-day period from the time of release at 24+1oC over a hatchery season. Results cover a 2-year period – differentiated by the shade of the data points. From M.M. Helm (1971) and previously unpublished data.