Effects of farming operations (grow-out)
Capture-based aquaculture implies the on-growing of selected species in captivity using “traditional” aquaculture practices. Typically the capture-based farmed species are enclosed in a controlled system, such as ponds or cages. In these they can be raised under suitable conditions, sheltered from predators and competitors, fed, and sometimes treated with medicaments to control diseases. The fish are confined at high densities and ideally supplied with all nutritional requirements. Thus, the more intensive the operation, the larger volume of wastes generated and potential impact on the local environment, and the greater the potential for disease.
Generally, intensive fish farming in cages generates a large amount of organic waste in the form of unconsumed feed, and faecal and excretory matter. This results in localized sediment build up with its attendant risks from self-pollution and disease, which may threaten the operations own sustainability. The particulate waste matter, originating from a single source, can accumulate in the sediments below or close to the farm, causing considerable organic and nutrient enrichment that may adversely affect benthic communities.
Major effects can be observed on the seabed and, to a lesser extent, on water quality. Careful site selection is critical for a successful, sustainable operation of all fish farms; special care is required in the case of marine aquaculture (Figure 130).

Figure 130. Philippines capture-based aquaculture (Photo: S. Fazi. Coastal Resource Management and Sustainable Tourism in Ulugan Bay Project implemented by (UNESCO/CSI/UNDP/Puerto Princesa City Government 2001)
Selecting a poor site may result in oxygen depletion in the bottom water, leading to the development of anoxic conditions in the sediment and the production of toxic gases such as hydrogen sulphide. These phenomena will adversely affect benthic organisms as well as the lower portion of the cages where the water is shallow. Furthermore, a reduction in dissolved oxygen level and an increase in Biochemical Oxygen Demand (BOD) and nutrients will occur in the water column around a fish farm, especially where the flushing rate and water exchange levels are too low for the biomass being raised. Other effects will also be observed, including an increase in suspended solids, chlorophyll and phaeopigment concentration, etc.
Usually, most fish farms have limited and localized environmental impacts associated with waste release; these are restricted to those areas in the immediate vicinity of the farm. In shallow waters, or in aquatic ecosystems with a poor water renewal rate, localized eutrophication around farms is possible. Polluted waters may result in fish disease and the mass mortality of cultured fish. The severity of environmental impacts depends upon the relationship between the intensity of fish culture operations and the water circulation/depth at the culture site (e.g. stocking density, feed input and the characteristics of the site). The level of sustainable production at any particular site is therefore a balance between the environmental waste loading (nutrient and organic matter) caused by the farm, and the renewal of the water at the site which prevents a build-up or significant change in the existing local environmental conditions. The use of mass balance equations and management mitigation measures can prevent this occurring. The most obvious changes caused by poorly located intensive marine culture systems for carnivorous fish in cages, are those to the local environment. These impacts, mainly on the benthos, may cause long term changes at sites in relatively quiescent waters, that may persist for many years after culture activity has ceased (Black 2001). Wu (1995) suggested that the degree of impact from the effluent wastes of cage aquaculture is dependent on the species, culture method and feed type, and on the nature of the receiving environment in terms of physics, chemistry and biology.
In the Mediterranean, the capture-based aquaculture of tuna is relatively new and so little is known about its environmental impact on the marine ecosystem. Tuna “farming”, among other activities, has been the target of criticism from environmental and other pressure groups due to the perceived impact of the industry on the environment (for an example of such views, see Tudela 2002a). A system that minimizes its impact, which removes uneaten food and consists of using a collecting net similar to that used in salmon farming, has proven to be successful (Agius 2002).
On the contrary, the impact of grouper capture-based aquaculture is clear, since there are lot of problems concerning water quality in the production areas (as also is the case for the yellowtails cultured in Japan). For example, water quality conditions are particularly poor in Hong Kong, parts of China, the Straits of Johore Bahru (Singapore/Malaysia) and the Philippines; in these locations fish densities are poorly managed, trash fish use is extensive, and there are problems with water contamination or low dissolved oxygen levels because of poor cage positioning. In the early 1990s, grouper production in Hong Kong was about 3 000 tonnes a year; in the last few years production has dropped to 1 000 tonnes a year due to several production and environmental problems (Sadovy 2000). There is an increasing interest in monitoring the environmental degradation from fish farming; thus, many countries have introduced tighter controls. Since 1999, Japan has introduced new legislation for the monitoring of sediment and water quality in fish farming areas, in order to assess sustainability (Pawar et al. 2001).
Advanced research efforts aimed at developing technical improvements in the systems used for eel farming are increasing, especially with regard to water quality, which has been a traditional problem in this type of culture (Gatta, Romagnoli and Venzi 2000). The problems to be addressed vary, depending on the species, area, stocking density, environmental capacity of the aquatic bodies, etc.
For the sake of simplicity, we have divided the main environmental effects of capture-based aquaculture into the following categories: feeding, organic pollution and eutrophication, effects of chemical use, algal blooms, benthos modification, and other interactions.
Feeding
Feeding in the capture-based aquaculture of most target species is mainly based on the use of trash fish, due to the inability of some of them to accept prepared diets. The use of trash fish has several disadvantages. There is a heavy dependency on the availability of locally caught fish for the production of the greater amberjack (S. dumerili) in Japan. The feeding of trash fish to densely cultured fish frequently results in deterioration of the environmental quality of receiving waters, leading to eutrophication and various fish diseases (Watanabe et al. 1991). Trash fish also have variable nutritional values and the potential for contamination due to being stored in poor hygienic conditions. In addition, its preparation (Figure 131) and storage require high labour inputs and specialized storage facilities (Shimeno 1991). Semi-moist diets are often prepared for feeding the greater amberjack in Japan through extruders mounted on board feeding boats; the ingredients comprise a mixture of “trash” fish with a premix of dry ingredients (M. New, pers. comm. 2003). Such diets are transferred to the cages in a flow of water. Extruded semi-moist pellets are also used (Figure 132). A photo of the feed delivery system to the cages is shown in Figure 139 in the following chapter of this report.
Worldwide, there is a general tendency to try to adopt the use of formulated diets; however, the use of trash fish still remains the norm in some areas, owing to conservative attitudes and/or economic reasons. When fish can be weaned onto a specialized prepared diet that replicates the normal nutritional intake, there is good evidence that they will perform much better than those fed on trash fish (Bell 1998). The existence of a link between high levels of feed wastage and the use of trash fish is still controversial, but current opinion favours the use of pellets (e.g. Black 2001; Chu 1999).

Figure 131. Trash fish used to feed capture-based farmed species in Asia (Source: NACA)

Figure 132. Dissection, showing pellets used for feeding capture-based farmed yellowtails (Photo: M. Nakada)
Waste feed from cage farming operations is always a problem. However, the particle size of that generated from the use of trash fish is much smaller than from prepared diets, leading to a wider dispersion and greater impact upon a larger area. In Hong Kong, marine fish culture activities are responsible for 3% of total BOD, 3% of total nitrogen loading, and 20% of total solid loading from domestic and industrial sewage discharges into the coastal waters. Of these cumulative pollution loadings 65% were derived from feed wastage alone (Chu 1999); trash fish is extensively used as feed in this location.
In some capture-based aquaculture (e.g. the culture of eels), fishmeal and fish oil are the key constituents of prepared feeds for intensive production (Tacon and Forster 2000). According to Black (2001) it requires 2 to 5 kg of captured fish to produce 1 kg of fish fed with a fishmeal based diet. There is a movement towards the substitution of fishmeal and fish oil by vegetable sources, in view of the limited supplies of marine feed ingredients (Goldbourg, Elliott and Naylor 2001; New and Wijkstrom 2002).
In terms of farm activities, feeding a formulated diet is easier than using trash fish, which demands more effort (buying, transporting, storage, thawing and chopping). However, when economic analyses are considered, feeding with trash fish may prove cheaper (Table 77), a factor that leads to understandable resistance to change, particularly amongst the operators of small grouper farms in SE Asia, for example (M. New, pers. comm. 2003).
Table 77. Comparative analysis of annual costs and returns per hectare of wild orange-spotted groupers (Epinephelus coioides) fed on alternative diets for 5 months in grow out ponds (Bombeo-Tuburan et al. 2001, modified)
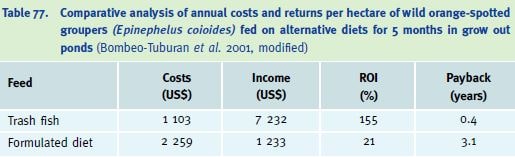
As a general rule, total dry diet costs should not exceed 25% of the farm-gate value of the cultured species (Tacon 1995). The formulated diet alone in the study of Bombeo-Tuburan et al. (2001) accounted for 39% of the total cost; thus from an economic viewpoint the formulated diet in this study was not suitable.
A good feed management system is essential for bluefin tuna farming. In the Murcia region of Spain, where the leading Mediterranean companies are located, the key environmental impacts are related to feeding and excretion. Impact due to feeding occurs because fish have a very poor FCR (up to 30:1). This depends on the water temperatures prevailing during the culture period. From this data it can be expected that there is a high degree of biological loading from waste materials (Belmonte Rios 2002). In a study in Croatia, approximately 6 200 tunas (average size 11.2 kg) were stocked in one cage, and fed daily at 5.08% of biomass, with a mixture mainly consisting (87.9%) of herrings (Clupea harengus L.), Sardina pilchardus (6.7%) and cephalopods (5.4%) (Katavic, Vicina and Franicevic 2003a).
In Port Lincoln, South Australia, farmed southern bluefin tuna are fed bait fish twice per day, six days per week. Feeding is generally done by placing frozen blocks of bait fish in a mesh cage within each pontoon (see Chapter 5, Figure 96). In 2001, 20 different species of bait fish were used (45 000 tonnes), which were sourced locally and overseas; FCRs averaged about 10- 15:1 (Clarke 2002). Poor FCR and the use of baitfish in capture-based tuna farming are major problems. Research is currently under way to develop manufactured feeds to replace this type of feeding. The demand from the tuna industry is creating increased fishing pressure on small pelagic fish stocks and some of these fisheries (e.g. anchovy), being poorly regulated, are showing signs of becoming depleted (Tudela 2002b).
Organic pollution and eutrophication
Most capture-based aquaculture is undertaken in cages, suspended in more or less open waters. Cage structures are relatively cheap, when compared with equivalent land-based facilities, and their use avoids the need for the expensive pumping of water, oxygenation and the removal of waste products. Water exchange and oxygenation are provided by the natural circulation of water through the cage system, as long as the cage is sited in a good open position. In Malta, for example, all tuna cages must be moored well away from the coast to avoid pollution risks to coastal zones. Marine coastal water quality is affected by various human activities (Figure 133) that cause discharges of organic matter, nutrients and other pollutants. The culture of fish in cages can contribute to the production of waste, which may stimulate and distort productivity and alter the abiotic and biotic characteristics of the ecosystem.

Figure 133. An example of poor water quality due to human impact (Photo: C. Silvestri)
Waste products from cage culture have a variety of sources: directly excreted, dissolved from feed and faecal particles, or released from particles that have been deposited on the seabed around the cages. Lipids originating from the diets used may form a film on the water surface, which is often observed around cages after feeding (Black 2001). The waste released from fish farms causes nutrient enrichment, the nutrients principally being carbon, nitrogen, phosphorus and silicon compounds. It is estimated that a large proportion of the phosphorus (85%), carbon (80-88%) and nitrogen (52-95%) compounds introduced into a marine fin fish culture system as feed may be lost to the environment as feed wastage, fish excretion, faeces production and respiration (Wu 1995). Feed wastage and excreted nitrogen can be significantly reduced when trash fish is replaced by pelleted feeds. Consequently, the use of trash fish is being regulated in some countries. Its use is banned in Denmark, where fish farms have to use formulated feeds (Chu 1999).
Results from various studies have shown that about 23% of the carbon, 21% of the nitrogen and 53% of the phosphorus from feed input may be accumulated in the bottom sediments, but that the significant impact is normally limited to an area within 1 km of the farm (Wu 1995). Organic matter accumulated on the seabed may lead to the development of an anoxic layer and reducing conditions in sediments, leading to the production of toxic gases (e.g. ammonia, methane and hydrogen sulphide). The toxicity of ammonia to marine life depends on pH, salinity and water temperature and the ability of the ammonia compounds to be lost to the air. The release of nitrate, nitrite and phosphorus may contribute to conditions that result in phytoplankton blooms. The majority of the nitrogen that is lost (50-60%) is in the dissolved form, directly from fish or by benthic fluxes from solid wastes beneath the cages; nitrogen losses from the fish are dependent on temperature and on dietary protein content (Buttle, Uglow and Cowx 1996).
Nitrogen is likely to be the limiting nutrient for phytoplankton growth in marine waters, while phosphorus is the limiting element for plant growth in fresh and brackish waters (Black 2001).
A nitrogen budget for the areolate grouper (Epinephelus areolatus) cultured under both laboratory and cage conditions has been reported (Leung et al. 1999). These workers attempted to balance the following equation:
C=G+M+E+F
where each term relates to a mass of nitrogen, and C is consumption, G is nitrogen retained for growth, M represents losses from mortality, E is excretory losses and F is losses through faeces. For a fish farm, the budget is represented by:
C=I-W
where I is the total input to the farm and W is the food wasted. Using a variety of techniques, Leung et al. (1999) were able to quantify each of these terms either by direct measurement or by difference. Figure 134 shows the budget derived for the cage farming of this species using trash fish as feed.
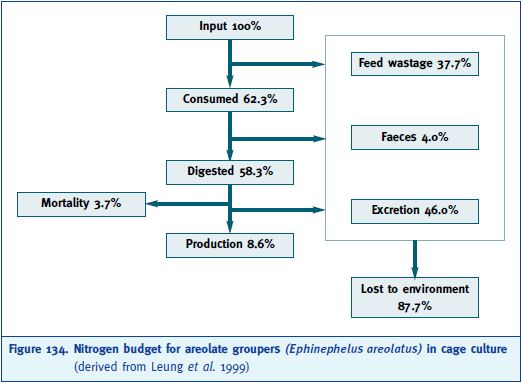
Figure 134. Nitrogen budget for areolate groupers (Ephinephelus areolatus) in cage culture (derived from Leung et al. 1999)
As shown in Figure 134, excretion of ammonia was the greatest contributor to nitrogen loss, followed by feed wastage, with faecal nitrogen loss being relatively unimportant. The nitrogen loss from this subtropical species fed trash fish is around three times greater than that for temperate species fed formulated diets (Leung, Chu and Wu 1999). Trash fish diets are inherently wasteful, as a consequence of their high nitrogen content and their tendency to break up and shed small unconsumed particles during feeding, and are thus major contributors to water quality degradation. Studies to assess the bio-energetic nutrient outputs from bluefin tuna capture-based aquaculture are in progress (Aguado and Garcia 2003). The budget for silicon compounds is not well known, even though it plays a key role in the metabolism of diatoms. There are continuing concerns that toxic dinoflagellate species may be promoted under nutrient imbalance conditions (Berry 1996). Holby and Hall (1994) proposed to maintain suitable ratios of silicon relative to nitrogen and phosphorus by placing cages in areas well supplied with fresh seawater and adding biogenic silica to the formula of the feed used.
The degree of impact from effluent wastes depends on husbandry parameters, including the species, culture method, and feed type, and on the nature of the receiving environment in terms of physics, chemistry and biology. The effect that dissolved wastes may have on the environment will also depend on the speed at which these nutrients are dispersed before being assimilated by the pelagic ecosystem. Cage structures are often located in areas of partially restricted exchange, because such locations generally provide shelter from extreme weather, thus protecting staff and equipment. In restricted exchange environments, it is essential to estimate flushing rates in order to asses the risks of significantly increasing nutrient concentrations in the immediate environment. Recent advances in our knowledge have greatly improved the situation in many areas. Several studies in various countries have calculated the cycling of nutrients; the loss of nutrients can be calculated by simple equations or by mass balance equations (Braaten 1992).
It is possible to minimize environmental impact by combining different tools in an impact assessment – the mass balance equation, the flushing rate, the characteristics of the site (depth, current, etc.) – of the farm. In Norway, a management system termed “modelling on-growing fish farm monitoring (MOM)” has been adopted, which combines simulation modellings of potential environmental impacts with a monitoring programme of increasing elaboration, dependent on the model’s predicted scale of impacts. The monitoring has to ensure each farm’s compliance with a set of required environmental quality standard (EQSs) (Pearson and Black 2000).
Effects of chemical use
Vitamins (e.g. vitamin B12 and biotin), minerals and pigments are often added to compounded feeds, but such additions are not common in the tropical and sub-tropical areas, where trash fish is mainly used as feed (e.g. for groupers, yellowtails). It is thought that the stimulating effects of vitamins and fish wastes have had an affect on the growth of red tide species, which have been reported in several studies (Wu 1995).
Therapeutants (such as malachite green, formalin, copper sulphate, etc.) and medicaments or antibiotics (e.g. aureomycin, oxytetracycline, terramycin, furazolidone, etc.) used to treat fish diseases are sometimes applied, and have resulted in the development of antibiotic resistant bacteria. The development of resistant bacterial pathogens of fish is well documented. For example, 100% bacterial resistance to oxytetracycline has been recorded from marine sediments near a fish farm after medication, which persisted for more than 13 months (Samuelsen, Solheim and Lunestad 1991).
Anti-foulants, typically copper based, are often used to treat cage netting to control fouling. There is a need for further research into the fate of releasing copper into the marine environment in this way, and its potentially polluting effects on planktonic and benthic flora and fauna (Mackay 1999).
Algal blooms
Toxic algal blooms have affected marine fish farming and will surely continue to do so. Algal blooms are mainly stimulated by the nutrient enrichment of waters (Figure 135), mainly by nitrogen and phosphorus compounds (Figure 136). When these blooms occur, there is a significant impact on the fish being cultured. Toxic species of the genera Chattonella, Heterosigma and Gymnodinium can cause massive fish kills and generate severe economic losses (Daranas et al. 2001; Fogg 2002; van den Bergh et al. 2002).
Figure 135. Bloom of dinoflagellates (Prorocentrum minimum) in Italian coastal waters, caused by organic pollution (x1 000) (Photo: P. Giordano)
Algal growth can also be accelerated by the presence of extraneous chemicals such as the use of vitamins in artificial feed. Biotin has been shown to stimulate the growth of certain toxic phytoplankton species (Gymnodinium aureoles); vitamin B12 can promote the alga Chrysocromulina polylepis (a “salmon killer”) and the dinoflagellate Heterosigma hakashiwo; while fish meat and faeces has been shown to stimulate the growth of red tide species such as Gymnodinium sp. and Chattonella antiqua (a “yellowtail killer”) in laboratory conditions (Wu 1995). Since 1957, large-scale red tides have been reported in the Seto Inland Sea of Japan. Since 1964, these phenomena, which are due to harmful marine phytoplankton, have spread throughout the whole area of the Inland Sea. In the summer of 1970, a red tide of Chattonella antiqua caused the death of 500 000 yellowtails, valued at ? 620 million. The capture-based aquaculture of yellowtails in the Seto Inland Sea has regularly suffered since that time from outbreaks of red tide due to Chattonella spp. The effect of Chattonella on the gills of the Japanese amberjack S. quinqueradiata has been studied: fish dying from Chattonella exposure show many types of lesions, while the gills of fish dying from environmental hypoxia show very few lesions. This demonstrated that the branchial oedema was induced by Chattonella and not by hypoxemia (Ono et al. 1998; Hishida, Ishimatsu and Oda 1999; Fogg 2002).
Along with the promotion of aquaculture, the establishment of monitoring systems for red tides and efforts to remove its causes are urgently needed.

Figure 136. High nutrient concentration causes algal blooms in coastal areas (Photo: C. Silvestri)
Benthos modification
Changes in macro-benthic community structure have been observed along a farm waste enriched gradient, with a decrease of the number of species. As detrital input increases, diversity also initially increases because there is the opportunity for the expansion of existing populations and the immigration of additional species. However, constant and long-term changes in water quality and physical and chemical conditions in the deeper level of sediments allow opportunistic species to proliferate, and longer lived species to disappear. Eventually, if input levels increase, the surface sediment becomes anoxic and only a small number of specialist taxa can survive (Mazzola, Mirto and Danovaro 1999; Mirto et al. 2000). A bio-indicator of polluted marine sediments is the polychaete Capitella sp., which is present in high abundance in these nutrient rich areas (Chareonpanich, Tsutsumi and Montani 1994).
Enrichment in organic matter, and the reduction of light due to fish farming and cages can cause a decline in sea-grass meadows (in the Mediterranean, the regression of Posidonia oceanica). Fish farming represents an additional anthropic disturbance on these already endangered sea grass communities. Recent studies (Delgado et al. 1997, 1999; Pergent et al. 1999; Ruiz, Perez and Romero 2001) show that P. oceanica disappears under fish cages, while surrounding areas are significantly degraded. This can be attributed to a direct reduction in light availability by the shading effect of the cages themselves and the high concentration of organic matter in sediments that is caused by the settling of particulate organic matter, such as uneaten food and faeces. Both factors are critical to sea-grass for survival (RuizRuiz, Perez and Romero 2001). Sea-grass decline can also continue after fish cage activity ceases; Delgado et al. (1999) reported a case in which the aquaculture activity ceased in 1991, yet the P. oceanica meadow declined until after 1994.
Studies to evaluate differences in the seabed community structure have been undertaken by Murcia University in the region of Murcia, Spain under tuna farming cages. These showed that the diversity of seafloor life beneath the cages is significantly lower than other areas, due to the inputs of organic matter from the feeding of the tuna (Marin et al. 2002).
Other interactions
Losses caused by predators to the fish farming industry itself are well documented. Whether by birds, seals, sea lions, carnivorous fish or even man (poaching), the potential commercial implications can be severe, not only in terms of fish loss, but in terms of the cost of the anti predatory methods necessary (nets, security personnel, etc.). Nash et al. (2000) considered such losses as a severe threat to aquaculture businesses. In Tasmania, Australia, 235 attacks by male fur seals on 15 farms producing Atlantic salmon and rainbow trout were recorded in 4 months, and one farm lost more than A$ 500 000 of fish in a single year. More recently, the loss by the industry to pinnipeds has been estimated by the Tasmanian Salmonid Growers Association at 2% over a 6-month period.
In the capture-based aquaculture of southern bluefin tuna a problem with mammal interactions exists as well. Marine mammals bite through the mesh and create holes in the nets of the cages, attacking the fish. The market-related risk is twofold: the potential loss of fish through the hole, and the reduction of the final quality of the harvested fish. Fish that have survived an attack (tuna could easily die from the stress) are badly scarred, loosing their full market value. In Port Lincoln, Australia, during the harvesting period, abalone divers have to cease all activities, since they face a high probability of meeting white pointer sharks, attracted by tuna blood and, probably, tuna stress movements in the cages. A few years ago, a white pointer shark was found inside a tuna cage. Apart from the production-related risk, it is necessary to consider the safety of the staff working on these aquaculture sites. Male sea lions have also been known to attack divers working on the nets. Sea lions generally bite or pull at the flippers of divers, but body bites have also been reported, particularly once the predator is inside the cage net (Nash et al. 2000).
Conclusions
The interaction of capture-based aquaculture with capture fisheries and traditional forms of aquaculture lead to potential environmental impacts, which are not always clearly defined or fully understood.
The impact on wild fish stocks as a result of catching wild “seed” is an important issue, but it is not easy to know the real status of stock resources. Knowledge of the effects of fishing on fish community dynamics is generally incomplete. Reasons for the decline of a resource cannot be evaluated without specific research, but even with scientific data it is often difficult to evaluate its status, because of other interacting effects (such as natural environmental events) that affect recruitment.
Where capture-based species are of significant commercial importance, it is more difficult to evaluate exactly the resource availability because other fishermen exploit the same stock, sometimes locally or, in the case of tuna (a highly migratory species), on a quite global scale. In many areas, high levels of fishing effort have caused increased recruitment overfishing of the life cycle of different species that are used in capture-based aquaculture, thus increasing their vulnerability to commercial extinction. Even “low level” artisanal fisheries can adversely affect stocks; a large proportion of the world’s species (e.g. grouper) are caught by traditional methods (Morris et al. 2000).
It is clear that there is a strong need for more and better data on the biology and fisheries of the target species used in capture-based aquaculture. This is essential if the sector is to survive alongside traditional fisheries and to serve as a basis complete enough to predict the sustainability of the industry. The main objective must be to achieve a degree of knowledge that could make the evaluation of the maximum sustainable yields (MSY) possible.
The capture of wild “seed” for this form of aquaculture is not the only issue to be considered as having potential negative interactions with the environment; habitat destruction, pollution, bycatch, feeding impact, etc., are all areas that also need more research to ensure the sustainability of the sector. The combination of these effects is difficult to understand owing to the lack of specific studies; this is particularly true for the new capture-based aquaculture species.
The importance of good feeding management should be highlighted. A common problem, found in the operation of all capture-based aquaculture activities, is the lack of specific diets that adequately match the nutritional requirements of each species. The palatability of such feeds is also of major importance. The extensive use of trash fish produces several environmental impacts and is one of the major sources of pollution around culture areas. The work now being undertaken to create and adopt formulated artificial diets could mitigate this problem. It is in the long-term interests of producers that successful specific research leading to the formulation and manufacture of commercial artificial feeds should be conducted, in order to maintain profitability and limit environmental degradation.
Finally, the effects of capture-based aquaculture on the environment can be significantly reduced by careful site selection, supported by good pre-site selection and pre-development appraisal of those farm locations that are envisaged, using modelling and other available assessment methods. Control of stocking densities, good feeding regimes, good health management and accurate environmental impact assessments (followed by continuous monitoring) should always be carried out.