DESCRIPTION OF THE SPECIES AND ITS USE IN AQUACULTURE
Biological outlines
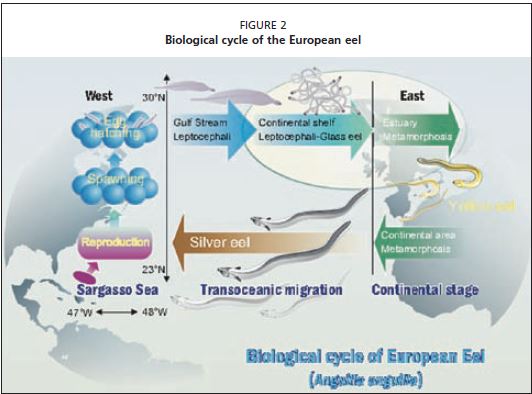
The European eel (Anguilla anguilla) occurs from Mauritania to the Artic Circle and the Mediterranean, and is an amphihaline and catadromous species with a complex biological life cycle, many aspects of which are still poorly understood or undocumented (Figures 1 and 2). For example, reproduction has never been observed and no eggs or spawning adults have been collected in the supposed spawning area which has been identified by Schmidt (1925) in the Sargasso Sea (Nilo and Fortin, 2001).
The taxonomic status of the species is still very vague and some hybridization between European (Anguilla anguilla) and American (Anguilla rostrata) eels has been observed (Boetius 1980; Avise et al., 1986; 1990). Regarded as a panmictic species, some recent papers hypothesise that the European eel is formed by 3 genetically differentiated sub populations (Wirth and Bernatchez, 2001; 2003).
However, recent work shows a strong intra-genetic variability that exceeds the inter genetic diversity among samples collected from various European stocks (Dannewitz
Figure 2
Biological cycle of the European eel
et al., 2005). This seems to indicate that the panmixia hypothesis is still valid and the results obtained by Wirth and Bernatchez (2001) could be an artefact linked to a meta population structure of the species (Maes et al., 2006; Pujolar, Maes and Volkaert., 2006).
Even in the absence of genetic structuring, there are physical, biological (particularly the diversity in the oceanic migration paths and intensity of estuarine recruitments) and socio-economic characteristics that make it possible to distinguish three geographical groups which produce silver eel populations with different mean age and growth attributes. The first is the “northern group” (North Sea and Baltic Sea) with low glass eel recruitment, producing silver eel with a slow growth rate that migrate towards the Sargasso Sea at a high mean age (Tesch, 1977). The exploitation of eels is focused primarily on the silver and yellow eel stages.
The second group is found in the Atlantic area from the British Islands to Portugal and is characterized by larger recruitment into the catchment areas. The biological cycles are of variable duration, from 5 to 15 years, and the sex ratio varies according to the physical and trophic characteristics of the habitat (Acou et al., 2004; Acou, 2006). The fishery mainly targets the glass eel stage, but some yellow and silver eel fisheries are well developed on certain rivers (e.g. Somme, Loire, Gironde) and along the littoral marshes of the Atlantic coast (Prouzet, 2002; 2003b). The third group, referred to as the “Mediterranean group”, is characterized by sparse glass eel recruitment. This group is more abundant than the one in the northern area, as demonstrated by the glass eel fisheries occurring in some Italian estuaries (Ciccotti, 2005). The biological cycles are often short and the stock is largely confined to coastal lagoons, particularly along the northern Africa and the French Mediterranean coasts. Exploitation is focused primarily on yellow and silver eels.
Fishing exploitation of the species
Eel is exploited at all its development stages and in various ecosystems (marine, brackish and freshwater). Fishing intensity on the different biological stages is highly variable according to the catchment areas and the geographical “groups” mentioned above. The exploitation of the glass eel ranges from 0 percent (e.g. in the Mediterranean Sea where fishing is prohibited in many river basins) to over 90 percent (Anonymous, 2002). In 2004, Dekker reviewed the fishing impact on the eel population and particularly on the glass eel stage indicating that the exploitation has reduced the abundance of the glass eel arriving at the mouth of the rivers observed by 85 percent (Dekker, 2004). However, studies conducted on several French rivers (mainly the Adour and Loire rivers) indicate that this level of impact is not usual. The estimated rate of exploitation is not higher than 15 percent (Bouvet, Prouzet and Bru, 2006) (Table 1). The first estimates collected on the Loire River on daily exploitation using push sieves indicate that the catch is lower than 30 percent (Prouzet et al., 2007). Considering that the glass eel is not exploited on many small rivers of the Atlantic coast and in many catchments located on the border of the Mediterranean, it is more realistic to consider that the global exploitation
TABLE 1
Estimates of the seasonal biomass of glass eel migrating upstream through the Adour estuary and mean rates of exploitation estimated during the recent fishing season (1998–2005)
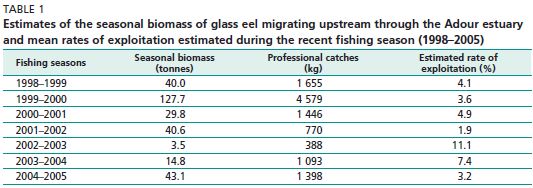
FIGURE 3
Typical glass eels caught in estuaries
rate is less than 50 percent. On the Adour River, which is free of dams in its estuary, surveys were carried out on the abundance of glass eel runs during the 1999–2000 fishing season, the best fishing year of the last decade (Prouzet, 2002; Lissardy et al., 2004). These studies showed that the total rate of exploitation by the push sieve fishery was 6.8 percent in the estuary, with a value lower than 6 percent one day out of two (Bru, Lejeune and Prouzet, 2004).
For yellow eel, the data show a large fluctuation of the exploitation rate according to the hydrological parameters. For example, the exploitation rate on the Ijsselmeer
Lake in Holland during the period 1989–1996 was estimated at 85 percent of all males and practically 100 percent of the females (Dekker, 2000). On the west coast of Sweden the escape of silver eel is estimated at 15 percent of the virgin stock (Svedang, 1999). At Grandlieu Lake in France the exploitation rate is estimated at 45–50 percent (Adam, 1997). This exploitation in many French rivers (e.g. Adour, Garonne and Dordogne rivers) is decreasing substantially due to the decline of the resource in many areas, but also due to the low interest among the young professional fishermen in this type of fisheries (Lissardy et al., 2004; Anonymous, 2004).
The fishing effort on the silver eels is also highly variable. There is no fishing along the French Atlantic coast (except for the Loire basin) and many rivers, but this is not the case in the Mediterranean where both the silver and yellow eels are targeted (Farrugio, Peyrille and Cabos, 2006; Melia et al., 2006; Prouzet and Nielsen, 2003). On the Loire River, Feunteun and Boisneau estimate an escape of between 80–90 percent from the professional fisheries (Anonymous, 2003). On the Irish Erne and Shannon rivers the escape level is on average higher than 60 percent, while less in the Baltic area where it is estimated at around 60 percent (Matthews et al., 200; McCarthy and Cullen, 2000; Moriarty, 1997).
Biological stages harvested
Yellow and silver eels are generally used for human consumption as is the glass eel in Spain and in the southwestern part of France. Glass eels, elvers and, more rarely, small yellow eels, are used for aquaculture and restocking. Glass eels are the most commonly used for aquaculture purposes for several reasons (Figure 3):
• almost 100 percent of glass eels accept the initial food offered;
• they are easier to wean on artificial food;
• they have been collected for direct consumption for many decades, and the fishing industry was able to provide a good supply when aquaculture activity started; • they are easy to transport; and
• compared to elvers they carry fewer pathogens, parasites, viruses or bacteria. Eels easily adapt to artificial conditions, as long as stress is avoided and glass eels only need a couple of days to get used to the artificial rearing conditions and will not attempt to escape as long as the conditions remain optimum. Food is offered to the newly introduced glass eels when the water temperature reaches 18–20 °C. The main types of food used at this early feeding stage are red worms (Tubifex tubifex) and cod roe (or crunched mussel) in Asian and European farms, respectively. Most of the glass eels quickly accept this food. Transition to artificial feed (i.e. paste and/or pellets) is progressive, gradually replacing the natural food with a nutritionally rich dry/artificial diet. Elvers are more difficult to wean onto artificial food, even when natural food is used to stimulate their appetite. An artificial feed with a pasty consistence is usually better accepted than pellets by wild-caught fingerlings. As only a few farmers currently base their production on elvers, the rest of this paper will deal exclusively with glass eel farming.
Difficulties in obtaining juveniles in controlled conditions
In contrast to the Japanese eel, Anguilla japonica, where the first glass eels were obtained in the laboratory in 2001 the success in artificial maturation of the European eel Anguilla anguilla has been limited until very recently (Tanaka et al., 2003). The first recorded hatched larvae were described in 1983 with the prolarvae surviving only 3.5 days (Bezdenezhnykh et al., 1983). In the EU “Reproduction of Eel I” project implemented from 2001 to 2003 several prolarvae hatched and survived for 2.5 days (Pedersen, 2003; 2004), while in the second phase of the same project (Reproduction of Eel II, 2005–2006), Tomkiewicz succeeded in hatching eggs from 18 female eels (personal communication). The number of hatched prolarvae from each female ranged from one to several thousands with the longest living prolarvae dying after 5 days. At this time the mouth was not open, indicating that the prolarvae probably died as a result of poor egg quality rather than from lack of food. These projects have shown how to produce European eel prolarvae and the next step is to produce higher quality eggs and to identify a suitable prolarvae feed. As a consequence of such technical difficulties, all the current production of glass eel comes from natural runs, primarily from the central colonization area, i.e. Bay of Biscay, south of the British Islands and from the Iberian Peninsula.
Farming techniques – a brief overview
Two rather different eel rearing techniques are in used: 1) the European intensive and 2) the Asian semi-intensive farming systems. A third farming technique also exists, used mainly in northern Italy and based on extensive farming in coastal brackish waters (known as “vallicoltura”), but this technique is hardly active any longer and is not addressed further in this paper (Ciccotti, 2005)1.
European intensive farming
This technique was developed to save on energy and wastewater costs and is mainly used in northern European countries (Figure 4). The eels are reared at very high densities (up to 120 kg eels/m3 of water) in indoor tanks with a strong water flow to provide the necessary oxygen and removal of waste products, such as ammonia, faecal matters, carbon dioxide and food remnants. The effluent is recycled in a specially designed unit. The water is unfit for a direct return to the culture tanks and is restored to proper physical and chemical standards, enabling the farmers to reuse the same water. Only 5–8 percent of the total farming water volume is renewed daily to avoid the build-up of toxic substances such as nitrates. A production unit with an annual output of 100 tonnes will have a daily renewal volume of approximately 60 m3. This highly sophisticated farming technique saves considerable water and energy, but it requires a highly trained and educated team of experts to run the facility. Furthermore, it requires a high investment and the overall farming risks are high as all the tanks are
1 After Ciccotti, 2005: “Up to the mid-1990s, Italy was the leading country in eel aquaculture, covering half of total European production, but today the Italian productive capacity and the market seem both to have reduced to about 1 500 tonnes per year. Currently, only a very small quota of the production comes from the extensive culture in the northern Adriatic (Valli) and in other coastal lagoons.”
FIGURE 4
An intensive indoor recirculated water eel farm in Europe (top) and an extensive outdoor still water eel pond in China (bottom)

interconnected. Most operations are automatic (e.g. feeding, grading, water parameter controls, cleaning) to save manpower. In fact, only 1.5 employees are needed for an annual production of 100 tonnes.
Asian semi-intensive farming As more than 50 percent of all European elvers collected since 1986 are farmed in Asia a brief description of the culture system is described below (Figure 4). Culture is usually carried out in still water ponds at considerably lower densities or a maximum of 20 kg/m?. Surface aerators provide the necessary oxygen and create a current which concentrates the sediment in the centre of the ponds. Water flushing, carried out twice daily, removes approximately 1/3 of the water volume and aids the removal of unwanted wastes and sediments. The waste water is usually discharged in a nearby stream. These farms occupy large areas and are located near freshwater streams as they require large volumes of water (approximately 4 000 m3 water/day/100 tonnes annual production). The culture ponds have a simple design usually separated by the water discharge channels. Most farm operations are conducted manually (feeding, grading, cleaning, etc.) and approximately 20–30 persons are employed for each 100 tonnes produced. Heating of the water during the cold winter months is carried out using a coal boiler. These farms have a very low technical level, poor sanitary monitoring, and do not require highly educated staff to operate and manage the system.
The main problems in eel farming are the following: 1) preventing escapes; 2) the significant percentage of fish refusing the artificial feeds; 3) disease problems; 4) high production costs; and 5) the slow growth in intensive farming systems once the fish has reached an average body weight of 150 grams.