9. Fish stock management
Recommendations on fish management issues such as nutritional needs, disease management, and handling, are similar, regardless of the culture system used (cages, ponds, tanks, etc.). For this reason, information that may not be strictly related to cage aquaculture is provided in this chapter.
BIOMASS MONITORING AND ASSESSMENT
The biological parameters analysed to evaluate the growth of fish are the same, regardless of the technology used. Fish reared in inland ponds or in offshore cages may be assessed using the same methods (for evaluating growth) and parameters (for growing conditions). Some of the most useful indices for analysis are described below, including FCR, SGR and condition index (CI).
Feed conversion ratio (FCR) is the ratio between the weight of feed fed to the fish and the weight gain of the fish. It is a measurement of how efficiently a fish converts feed into body mass (i.e. weight gain). For example, an FCR of 2.8 means that 2.8 kg of feed is required to produce 1 kg of live fish weight.
where:
FCR = ?Feed /(W1- Wo)
? Feed is the quantity of feed distributed during a set time interval to t1; W is the fish biomass at to (start period);
W is the fish biomass at t (end period).
Specific growth rate (SGR) is a measure of how quickly the fish grows over a certain time interval. Fish growth, measured as weight gain, rarely follows a linear pattern and, therefore, the natural log (In) is used to transform the data.
where:
SGR = 100 ((In W1 - In Wo)/(ti-to))
t-to is the time period (number of days);
W0 is the biomass at to
W1 is the biomass at t.
Condition factor (K) or condition index (CI) is a metric to determine how heavy a fish is for its length. Fish gain weight faster than they gain length, essentially becoming "fatter" as they get longer. The condition factor provides a way to compare the relative health of groups of fish. Different species will have a different exponent, but most have one of about three.
K=W/SL3
where:
Wis the weight of fish;
SL is the standard length of the fish.
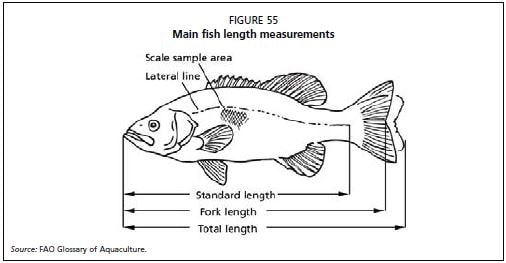
Standard length is measured from the tip of the mouth of the fish to the base of the tail, and does not include the caudal fin, which can be variable in length. Figure 55 provides the most frequently-used fish measurements. Consistency in the method of measurements is important in order to compare the results between cohorts and over time, so all staff should be trained on exact methods of measurement.
Tracking cages and cohorts
Traceability can only be assured on a cage farm if the information recorded is organized according to cohorts and not by cages. Cage numbering should refer to each cage’s position in the mooring grid, while cohorts of fish may be moved from one cage to another during the production cycle.
A unique batch ID code must be assigned to each new cohort of fish that is introduced into the cage farm. This code will be used for recording all pertinent information to ensure traceability. Table 36 provides an example of batch coding. A two letter species code is assigned to each reared species and then each batch number will contain the species code (two letters), the year of stocking (two numbers) and a following number for each batch of that species. The example below refers to the batch SB1105, which is the fifth batch of sea bream stocked in the farm on 2011.
TABLE 36
Example of fish cohort numbering
![]()
Fish stock report
For stock management, a stock report will provide the basic control tool of the different fish batches. The report should include all of the most relevant biological parameters and batch information that could prove useful for a quick evaluation of the status of each batch.
Each report refers to a specific period, either a week (preferred) or a month, and the information refers to each batch.
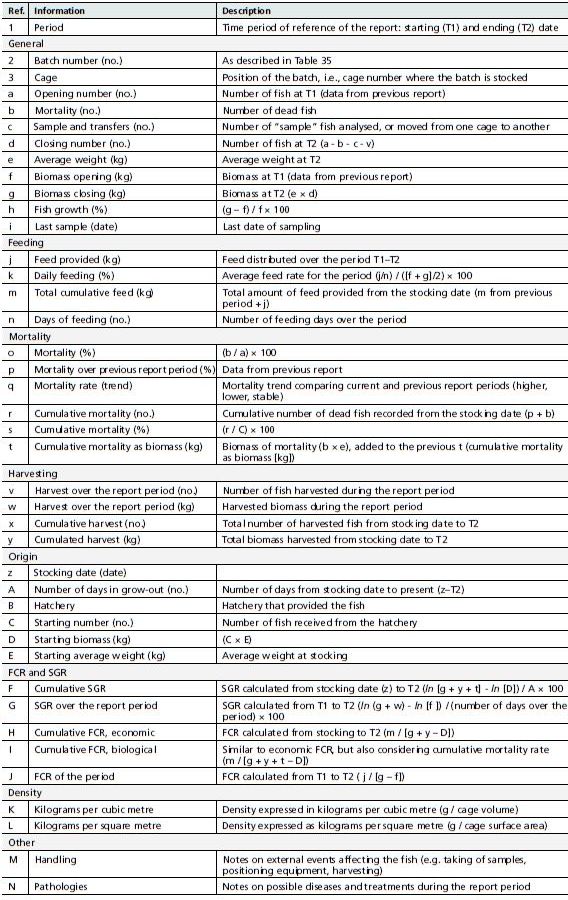
A comprehensive stock report would include the type of information shown in Table 37.
The report can also include information on any fish samples taken during the report period. Information for each sampling should include: batch number, date, number of sampled fish, expected average weight, actual observed calculated weight, coefficient of variation (if sampled individually).
TABLE 37
List of information to be included in the periodic fish stock report for management control for each fish batch
Fish sampling
It is strongly recommended that farmers periodically sample each fish cohort to check the growth rate and make observations on fish health. When taking a sample of fish, several different approaches may be taken, such as: 1. Live sampling at cage-side. Catch the fish, make the observations on board the farm boat, and then release the fish back into the cage (see Plates 115-120). This is the most time-efficient method, but the boat must be equipped with a hanging scale, a tank (possibly including an aeration system), small containers to weigh the fish, a fishing net and a hand-scoop net.
The procedure is as follows:
Boat is moored onto the side of the cage. A larger group of fish (up to one tonne) are caught with the seine net (Plate 115). A diver can help trap the fish, ensuring that the sampled fish are representative of the entire cage population (including the smaller or weaker fish that are often found towards the bottom of the cage).
A small number of fish (about 200- 300 individuals) are carried to the tank on board with the hand-scoop net (Plates 116 and 117). If the fish are overly sensitive to being handled, a dose of anaesthetic can be added into the tank. The tank can be provided with an internal net that can be lifted to collect fish quickly when a few fish are left in the tank at the end of the operation.
Fish are weighed and released back into the cage (Plates 118-120). This operation can be done on single fish or groups of fish. The latter option is quicker, but does not provide information on size variation. This information may become relevant if the batch is soon to be harvested and sold, and information on size variation is
required. 2. Live sampling on shore. The general method is to catch the fish, transfer the fish to a land- based facility, conduct the measurements on land, and then return the fish to the cage. This alternative method is advisable if the sea state is rough, which can cause the scales to give inaccurate readings. The procedure is very similar to the methods described above, except that in this case, two tanks will be needed, both of which must be equipped with

an aeration system. On the jetty, the scale should be positioned near the boat. The fish are then moved from one tank to the other after each weighing on land. 3. Terminal sampling. A sample of fish is collected, killed, and then taken to the land- based unit for measurements on land. This option is preferable if a closer inspection of the fish is desirable. In this case, the length and weight of each fish can be measured accurately. Moreover, a pathology inspection can be conducted on-site or at a specialized laboratory.
Fish can be sampled as described above, and then killed by thermoshocking them in a tank filled with seawater and ice slurry (see Chapter 10). If analysis of the fish is to include monitoring parasites, some sample specimens. should be killed with a percussive stunning, using an appropriate tool such as a heavy wooden handle or a metallic wand to deliver a

concussive blow to the head in the area just above the eyes (the area adjacent to the brain). Percussive stunning is necessary because ectoparasites may detach from the host in the water-ice slurry. Always keep the fish refrigerated after stunning.
Note: A number of chemicals are available as fish anaesthetic drugs, such as 2-phenossietyhand, clove oil, and MS-222. Concentration levels and exposure of the fish to the drug vary depending on the species, the water temperature and the level of sedation required; furthermore, the chemicals may be approved or not by local regulations, therefore only approved drugs should. be taken into consideration. Be sure that the tank is drained, washed and refilled with clean seawater after sampling from each cage, to prevent contamination of parasite samples.
